Effects of Mixing Speed and Mixing Time on Powder Segregation During Powder Mixing for Binder Jetting Additive Manufacturing: An Experimental Study
Abstract
1. Introduction
2. Materials and Methods
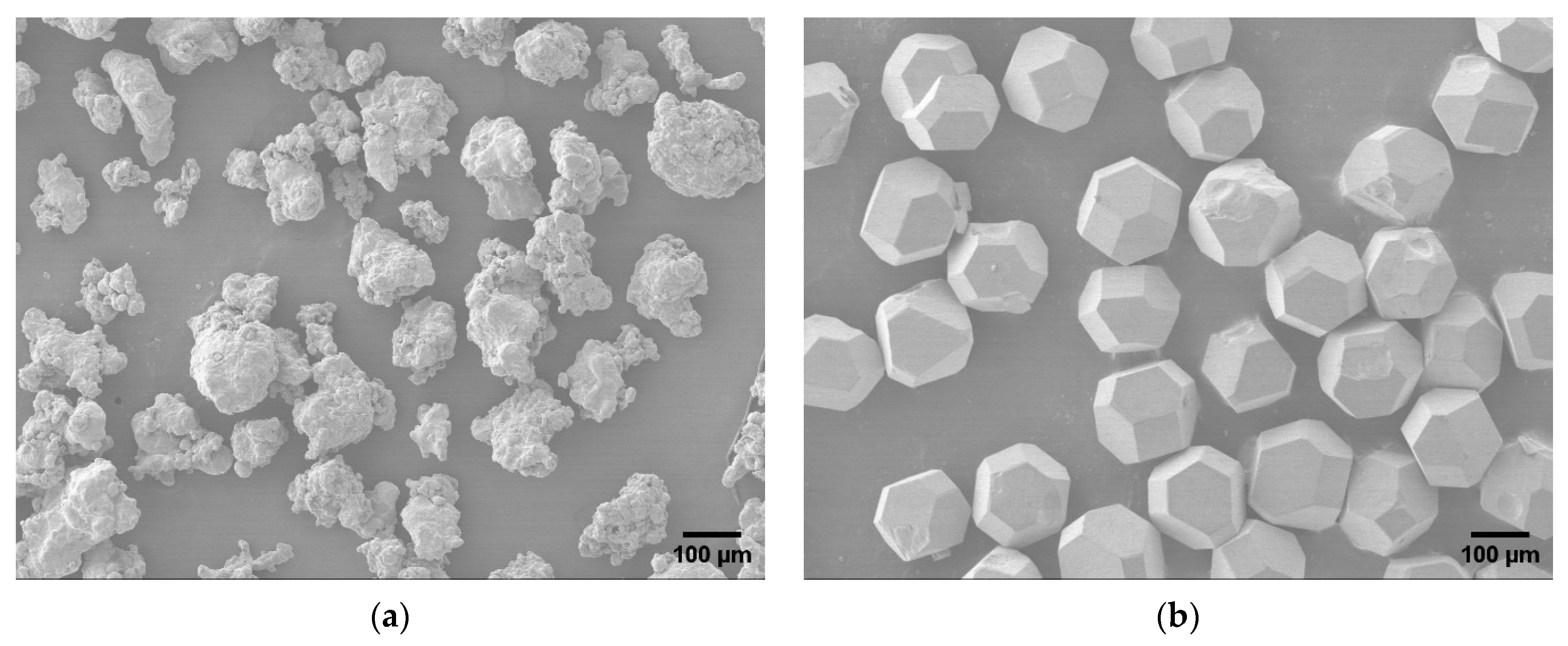
2.1. Materials
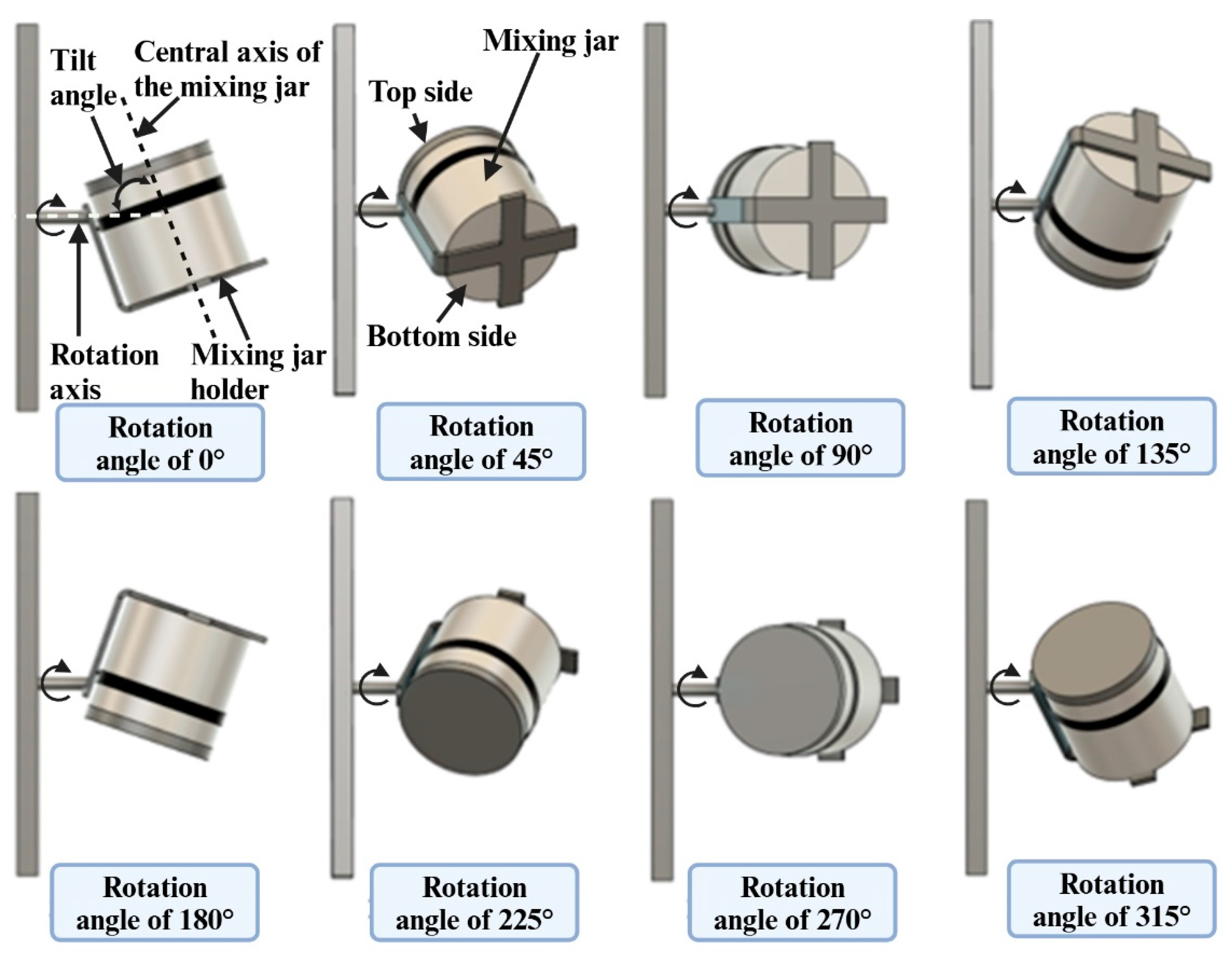
2.2. Mixing Equipment
2.3. Design of Experiments
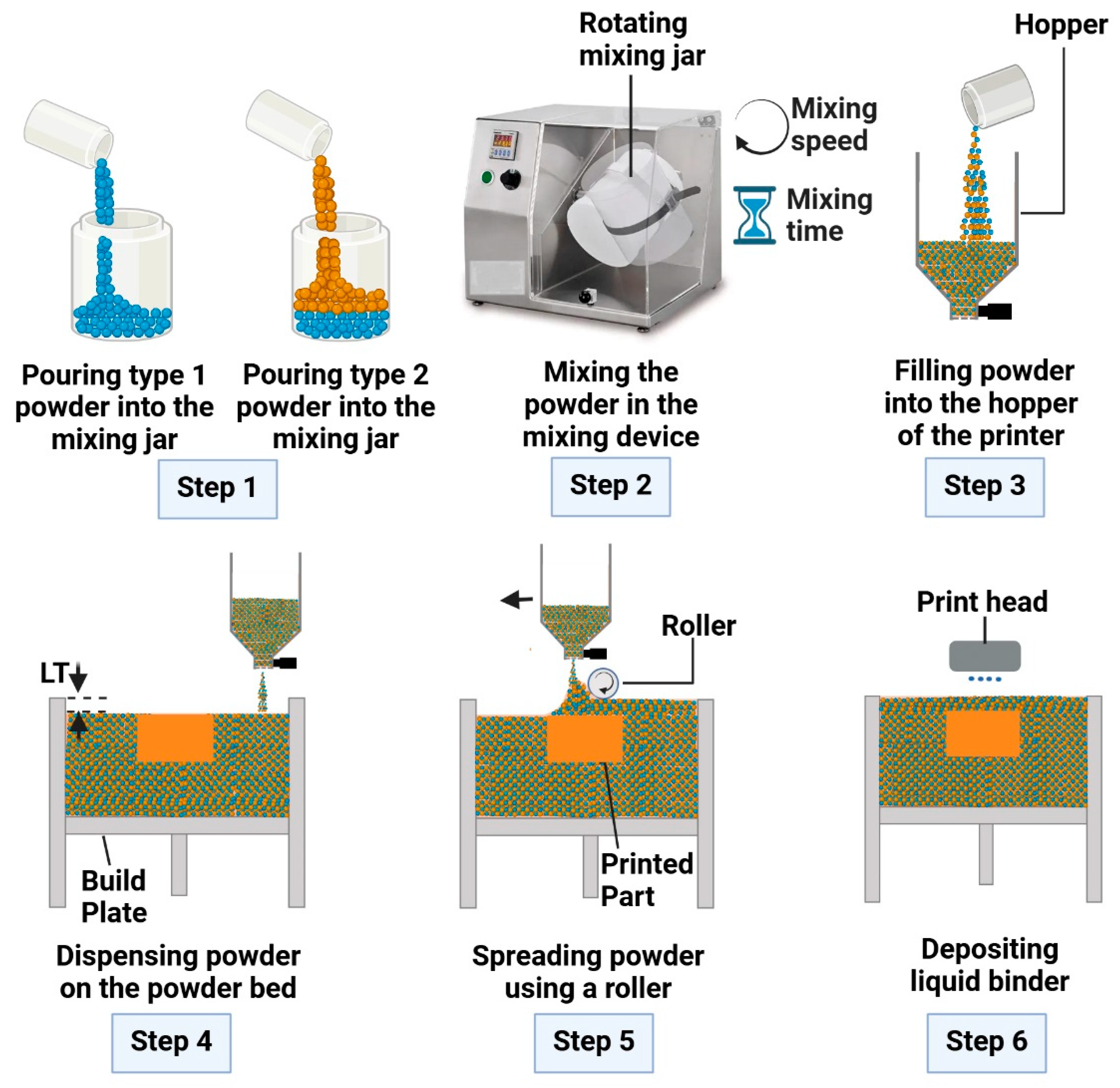
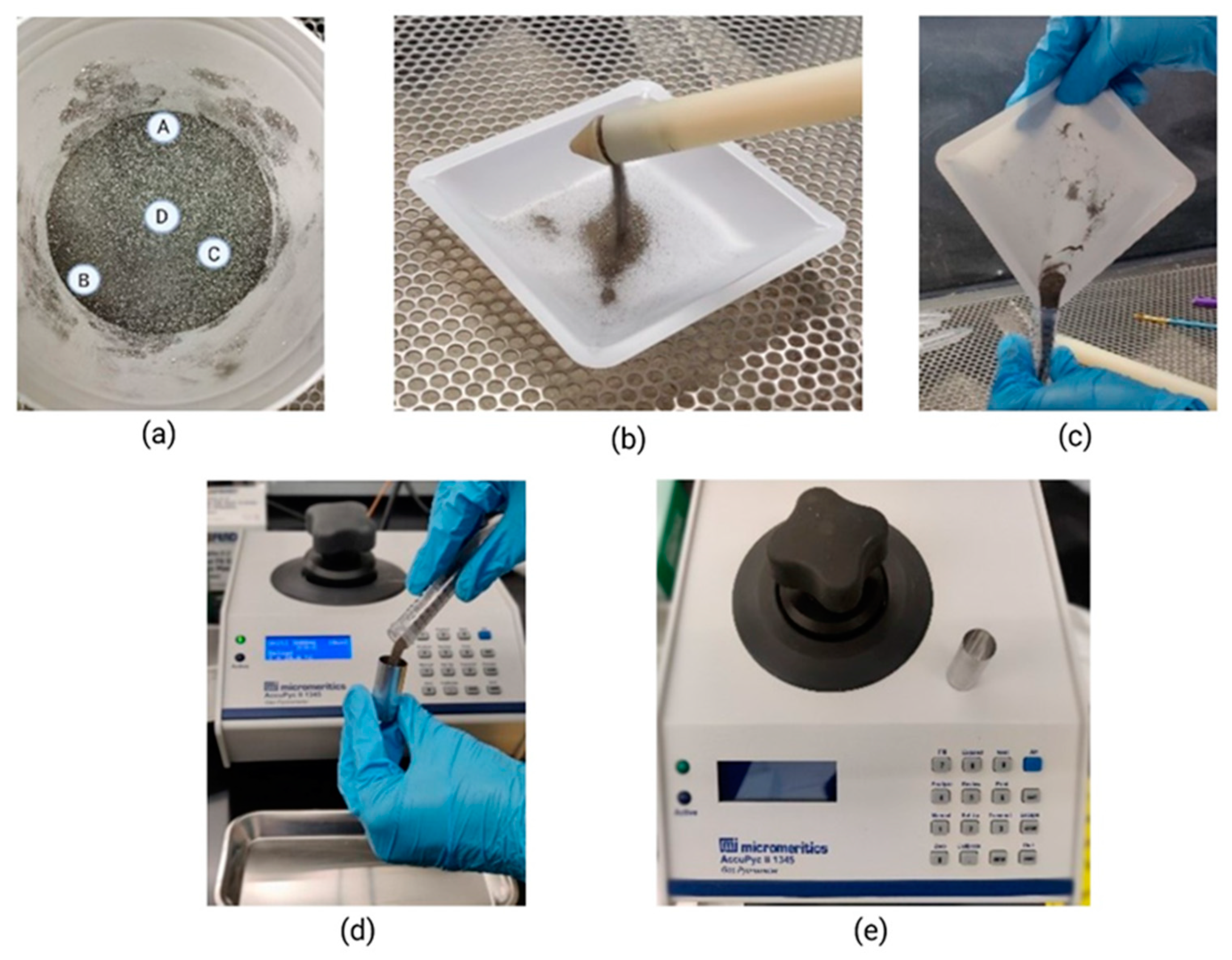
2.4. Procedures of Powder Mixing, Sampling, and Density Measurement
2.5. Evaluation of Segregation
3. Results and Discussion
3.1. Main Effect of Mixing Speed
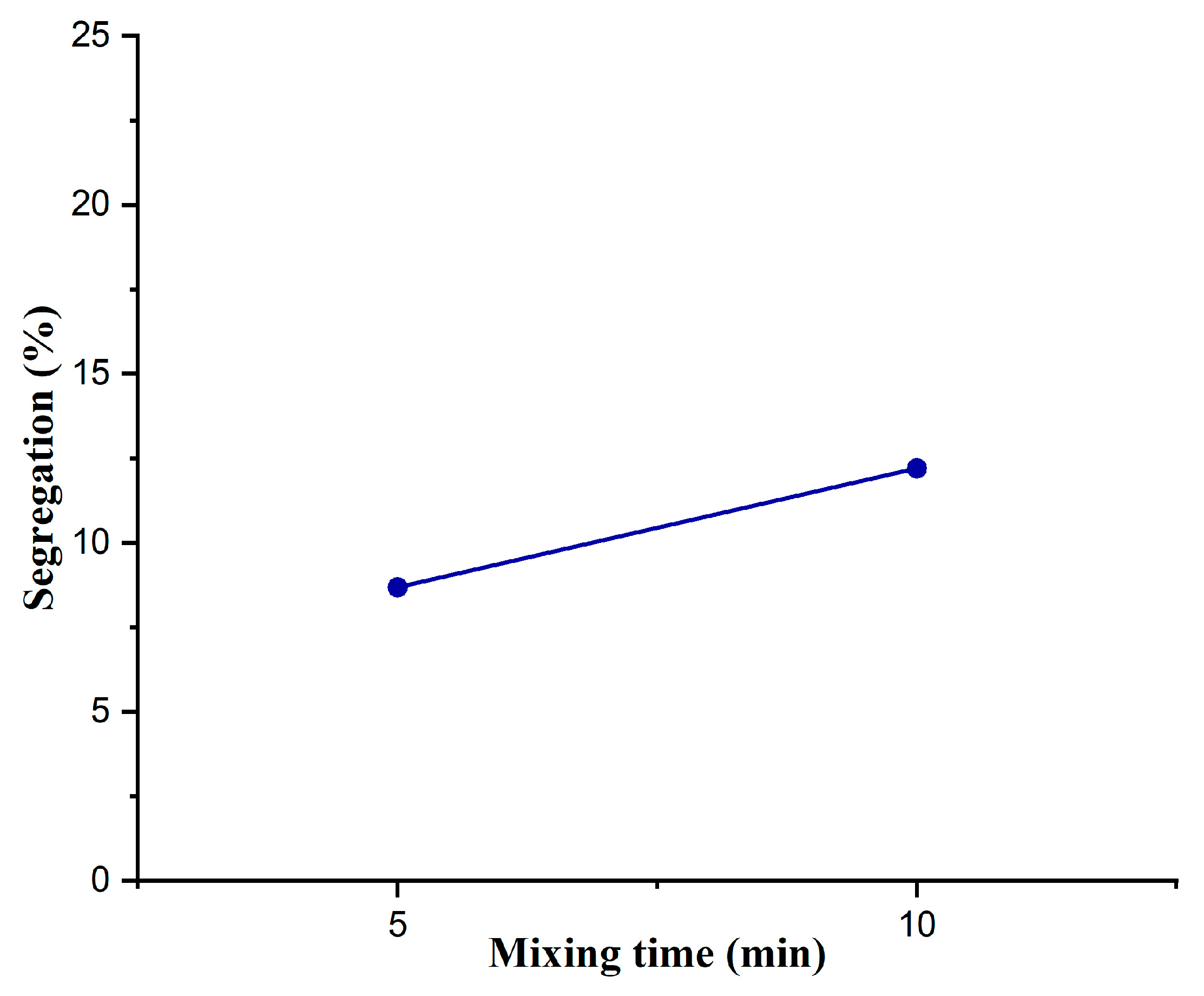
3.2. Main Effect of Mixing Time
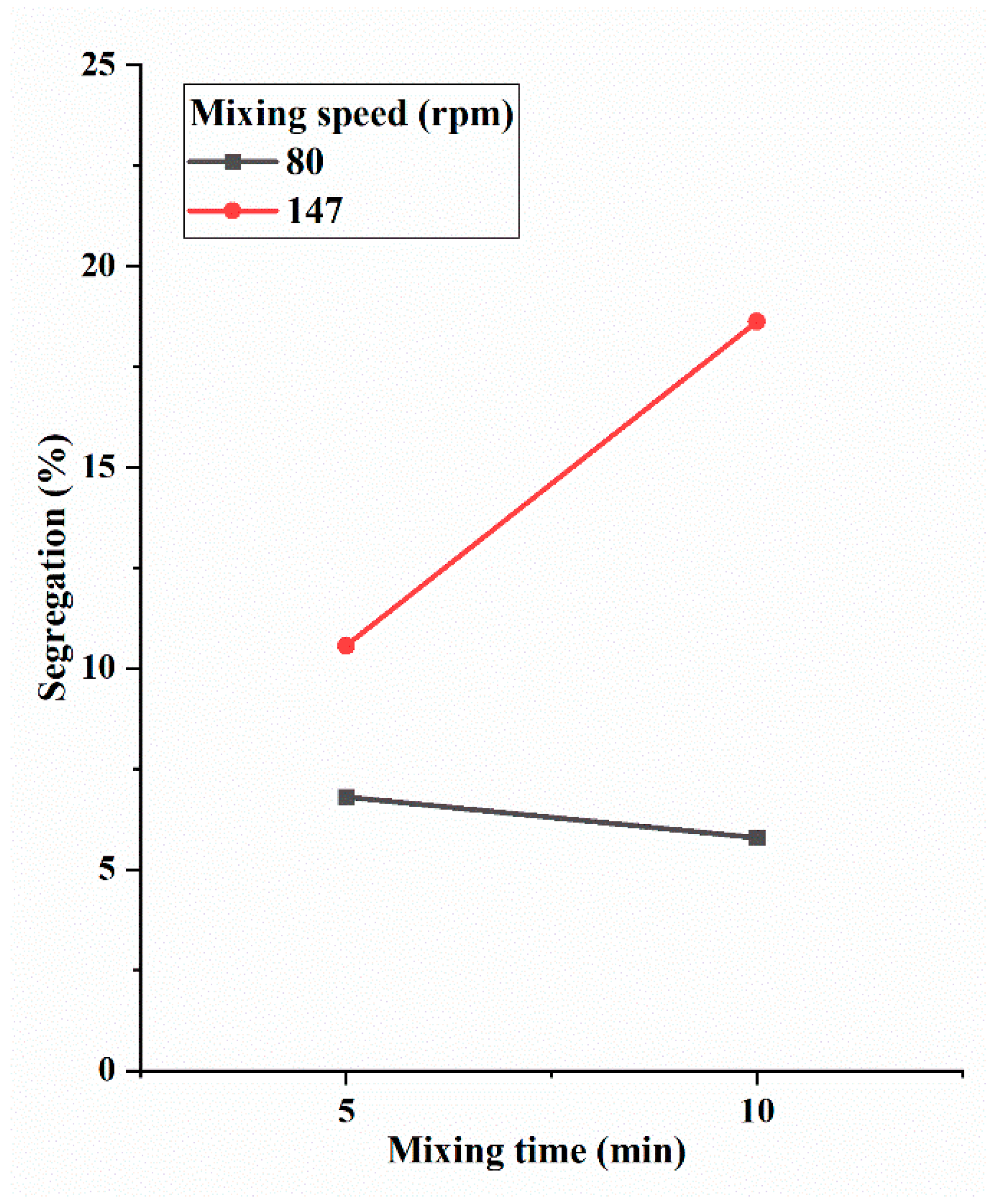
3.3. Interaction Effect of Mixing Speed and Mixing Time
4. Conclusions
- Powder segregation was higher at the high level of mixing speed (statistically significant at the significance level of 0.03).
- Powder segregation was higher at the high level of mixing time (statistically significant at the significance level of 0.30).
- At the low level of mixing speed, longer mixing time reduced powder segregation; however, at the high level of mixing speed, longer mixing time increased powder segregation (statistically significant at the significance level of 0.19).
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- ASTM52900-15; Standard Terminology for Additive Manufacturing—General Principles—Terminology. ASTM International: West Conshohocken, PA, USA, 2015; Volume 3, p. 5.
- Miao, G.; Du, W.; Pei, Z.; Ma, C. A literature review on powder spreading in additive manufacturing. Addit. Manuf. 2022, 58, 103029. [Google Scholar] [CrossRef]
- Moghadasi, M.; Jaraczewski, J.; Mahdaviarab, A.; Pei, Z.; Ma, C. Roller-compaction-assisted binder jetting of textured ceramics. J. Manuf. Process. 2024, 124, 240–247. [Google Scholar] [CrossRef]
- Mostafaei, A.; Elliott, A.M.; Barnes, J.E.; Li, F.; Tan, W.; Cramer, C.L.; Nandwana, P.; Chmielus, M. Binder jet 3D printing—Process parameters, materials, properties, modeling, and challenges. Prog. Mater. Sci. 2021, 119, 100707. [Google Scholar] [CrossRef]
- Moghadasi, M.; Miao, G.; Li, M.; Pei, Z.; Ma, C. Combining powder bed compaction and nanopowders to improve density in ceramic binder jetting additive manufacturing. Ceram. Int. 2021, 47, 35348–35355. [Google Scholar] [CrossRef]
- Li, M.; Huang, J.; Fang, A.; Mansoor, B.; Pei, Z.; Ma, C. Binder jetting additive manufacturing of copper/diamond composites: An experimental study. J. Manuf. Process. 2021, 70, 205–213. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.; Zhang, S.; Zou, S.; Cheng, T.; Zhu, D. Effect of bimodal powder on densification and mechanical properties of 316L stainless steel fabricated by binder jet 3D printing. J. Mater. Res. Technol. 2023, 27, 4043–4052. [Google Scholar] [CrossRef]
- Du, W.; Roa, J.; Hong, J.; Liu, Y.; Pei, Z.; Ma, C. Binder jetting additive manufacturing: Effect of particle size distribution on density. J. Manuf. Sci. Eng. 2021, 143, 091002. [Google Scholar] [CrossRef]
- Du, W.; Ren, X.; Pei, Z.; Ma, C. Ceramic binder jetting additive manufacturing: A literature review on density. J. Manuf. Sci. Eng. 2020, 142, 040801. [Google Scholar] [CrossRef]
- Mindt, H.W.; Megahed, M.; Lavery, N.P.; Holmes, M.A.; Brown, S.G.R. Powder Bed Layer Characteristics: The Overseen First-Order Process Input. Metall. Mater. Trans. A 2016, 47, 3811–3822. [Google Scholar] [CrossRef]
- Alizadeh, E.; Bertrand, F.; Chaouki, J. Discrete element simulation of particle mixing and segregation in a tetrapodal blender. Comput. Chem. Eng. 2014, 64, 1–12. [Google Scholar] [CrossRef]
- Pereira, G.; Cleary, P. Segregation due to particle shape of a granular mixture in a slowly rotating tumbler. Granul. Matter 2017, 19, 23. [Google Scholar] [CrossRef]
- He, S.Y.; Gan, J.Q.; Pinson, D.; Zhou, Z.Y. Particle shape-induced radial segregation of binary mixtures in a rotating drum. Powder Technol. 2019, 341, 157–166. [Google Scholar] [CrossRef]
- Liu, R.; Yin, X.; Li, H.; Shao, Q.; York, P.; He, Y.; Xiao, T.; Zhang, J. Visualization and quantitative profiling of mixing and segregation of granules using synchrotron radiation X-ray microtomography and three dimensional reconstruction. Int. J. Pharm. 2013, 445, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Mayer-Laigle, C.; Gatumel, C.; Berthiaux, H. Mixing dynamics for easy flowing powders in a lab scale Turbula® mixer. Chem. Eng. Res. Des. 2015, 95, 248–261. [Google Scholar] [CrossRef]
- Liao, C.-C. A study of the effect of liquid viscosity on density-driven wet granular segregation in a rotating drum. Powder Technol. 2018, 325, 632–638. [Google Scholar] [CrossRef]
- Gürmeriç, V.; Doğan, M. A new approach to evaluate mixing time in a V-type powder mixer: Taguchi weighted grey relational analysis. Eur. Food Res. Technol. 2024, 250, 547–564. [Google Scholar] [CrossRef]
- Pasha, M.M.; Arman, M.S.; Khan, F.; Pei, Z.; Kachur, S. Effects of Layer Thickness and Compaction Thickness on Green Part Density in Binder Jetting Additive Manufacturing of Silicon Carbide: Designed Experiments. J. Manuf. Mater. Process. 2024, 8, 148. [Google Scholar] [CrossRef]
- Pei, Z.; Strasbaugh, A. Fine grinding of silicon wafers: Designed experiments. Int. J. Mach. Tools Manuf. 2002, 42, 395–404. [Google Scholar] [CrossRef]
- Yap, Y.L.; Wang, C.; Sing, S.L.; Dikshit, V.; Yeong, W.Y.; Wei, J. Material jetting additive manufacturing: An experimental study using designed metrological benchmarks. Precis. Eng. 2017, 50, 275–285. [Google Scholar] [CrossRef]
- Rahman, T.T.; Arman, M.S.; Perez, V.; Xu, B.; Li, J. Analysis of the operating conditions of pulse electric field–assisted EHD for sodium alginate printing using design of experiment approach. Int. J. Adv. Manuf. Technol. 2021, 115, 2037–2047. [Google Scholar] [CrossRef]
- DeVor, R.E. Statistical Quality Design and Control: Contemporary Concepts and Methods; Chang, T.-H., Sutherland, J.W., Eds.; Pearson/Prentice Hall: Upper Saddle River, NJ, USA, 2007. [Google Scholar]


| Powder Type | Result | Reference |
|---|---|---|
| Sucrose and starch spherical powder (particle size of 2 and 4 mm, respectively) | Higher mixing speed increased segregation | [11] |
| Spherical and cubical powder (particle size of 2 mm) | Higher mixing speed increased shape-induced segregation | [12] |
| Ellipsoid and spherical-shaped powder (particle size of 3 mm) | Higher mixing speed reduced shape-induced segregation | [13] |
| Microcrystalline cellulose spherical powder (particle size of 0.55 mm) and starch non-spherical powder (particle size of 1.90 mm) | Longer mixing time reduced size and shape-induced segregation | [14] |
| Lactose powder (particle size of 65.4 µm) and couscous powder (particle size of 1 mm) | Longer mixing time reduced segregation | [15] |
| Glass and polypropylene beads of same size (3 mm) | As mixing time increased, segregation reduced (till mixing time reached a certain value) and then did not change much | [16] |
| Powder A | Powder B | |
|---|---|---|
| Material type | Metal | Ceramic |
| Average particle size (µm) | 118 | 230 |
| Particle shape | Irregular | Diamond |
| True density (g/cm3) | 7.93 | 3.86 |
| Variable | Low Level (−) | High Level (+) |
|---|---|---|
| Mixing speed (rpm) | 80 | 147 |
| Mixing time (min) | 5 | 10 |
| Experiment Order | Mixing Speed | Mixing Time |
|---|---|---|
| 1 | − | − |
| 6 | − | − |
| 10 | − | − |
| 3 | + | − |
| 5 | + | − |
| 7 | + | − |
| 2 | − | + |
| 8 | − | + |
| 11 | − | + |
| 4 | + | + |
| 9 | + | + |
| 12 | + | + |
| Experiment Order | Density at Each Location (g/cm3) | Mean Density (g/cm3) | Density Deviation at Each Location (%) | Segregation (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | A | B | C | D | |||
| 1 | 5.79 | 6.00 | 5.64 | 5.79 | 5.81 | −0.26 | 3.36 | −2.84 | −0.26 | 6.20 |
| 6 | 5.60 | 5.79 | 5.89 | 5.66 | 5.74 | −2.35 | 0.96 | 2.70 | −1.31 | 5.06 |
| 10 | 5.73 | 5.35 | 5.87 | 5.79 | 5.69 | 0.79 | −5.89 | 3.25 | 1.85 | 9.15 |
| 3 | 5.93 | 6.16 | 6.5 | 6.23 | 6.21 | −4.43 | −0.73 | 4.75 | 0.40 | 9.19 |
| 5 | 5.41 | 5.64 | 6.28 | 5.74 | 5.77 | −6.20 | −2.21 | 8.89 | −0.48 | 15.08 |
| 7 | 6.01 | 6.27 | 5.86 | 5.82 | 5.99 | 0.33 | 4.67 | −2.17 | −2.84 | 7.51 |
| 2 | 5.73 | 5.8 | 5.87 | 5.60 | 5.75 | −0.35 | 0.87 | 2.09 | −2.61 | 4.70 |
| 8 | 5.71 | 5.61 | 5.91 | 5.65 | 5.72 | −0.17 | −1.92 | 3.32 | −1.22 | 5.24 |
| 11 | 5.62 | 5.56 | 5.98 | 5.76 | 5.73 | −1.92 | −2.97 | 4.36 | 0.52 | 7.33 |
| 4 | 6.76 | 6.71 | 5.86 | 5.61 | 6.24 | 8.42 | 7.62 | −6.01 | −10.02 | 18.44 |
| 9 | 5.30 | 4.93 | 6.56 | 5.97 | 5.69 | −6.85 | −13.36 | 15.29 | 4.92 | 28.65 |
| 12 | 5.46 | 5.74 | 5.87 | 5.97 | 5.76 | −5.21 | −0.35 | 1.91 | 3.65 | 8.85 |
| Mixing Speed | Mixing Time | Segregation Mean (%) | Standard Deviation (%) |
|---|---|---|---|
| − | − | 6.80 | 2.11 |
| − | + | 5.76 | 1.39 |
| + | − | 10.59 | 3.98 |
| + | + | 18.65 | 9.90 |
| DF | Sum of Squares | Mean Square | F Value | p Value | |
|---|---|---|---|---|---|
| Mixing speed | 1 | 206.687 | 206.687 | 6.908 | 0.030 |
| Mixing time | 1 | 37.249 | 37.249 | 1.245 | 0.297 |
| Interaction | 1 | 61.817 | 61.817 | 2.066 | 0.189 |
| Model | 3 | 305.752 | 101.917 | 3.406 | 0.074 |
| Error | 8 | 239.371 | 29.921 | ||
| Total | 11 | 545.123 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasha, M.M.; Pei, Z.; Arman, M.S.; Gasdaska, C.J.; Kao, Y.-T. Effects of Mixing Speed and Mixing Time on Powder Segregation During Powder Mixing for Binder Jetting Additive Manufacturing: An Experimental Study. J. Manuf. Mater. Process. 2025, 9, 117. https://doi.org/10.3390/jmmp9040117
Pasha MM, Pei Z, Arman MS, Gasdaska CJ, Kao Y-T. Effects of Mixing Speed and Mixing Time on Powder Segregation During Powder Mixing for Binder Jetting Additive Manufacturing: An Experimental Study. Journal of Manufacturing and Materials Processing. 2025; 9(4):117. https://doi.org/10.3390/jmmp9040117
Chicago/Turabian StylePasha, Mostafa Meraj, Zhijian Pei, Md Shakil Arman, Charles J. Gasdaska, and Yi-Tang Kao. 2025. "Effects of Mixing Speed and Mixing Time on Powder Segregation During Powder Mixing for Binder Jetting Additive Manufacturing: An Experimental Study" Journal of Manufacturing and Materials Processing 9, no. 4: 117. https://doi.org/10.3390/jmmp9040117
APA StylePasha, M. M., Pei, Z., Arman, M. S., Gasdaska, C. J., & Kao, Y.-T. (2025). Effects of Mixing Speed and Mixing Time on Powder Segregation During Powder Mixing for Binder Jetting Additive Manufacturing: An Experimental Study. Journal of Manufacturing and Materials Processing, 9(4), 117. https://doi.org/10.3390/jmmp9040117







