Effect of T6 Tempering on the Wear and Corrosive Properties of Graphene and B4C Reinforced Al6061 Matrix Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials Used
2.2. Methodology
2.3. Characterization Techniques
3. Result and Discussion
3.1. Wear Behaviour
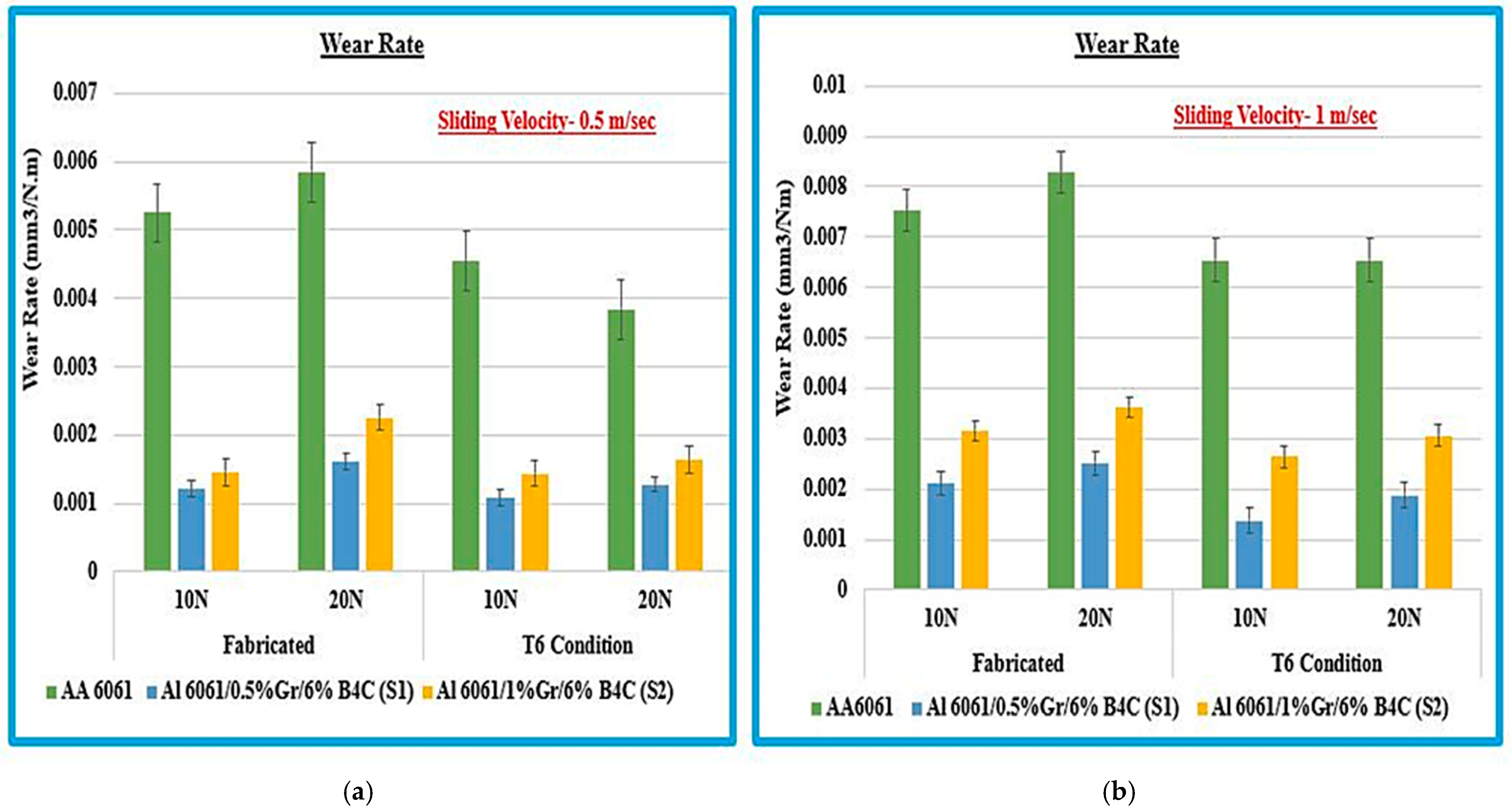
3.1.1. Effect on the Wear Rate
3.1.2. Effect on Mass Loss
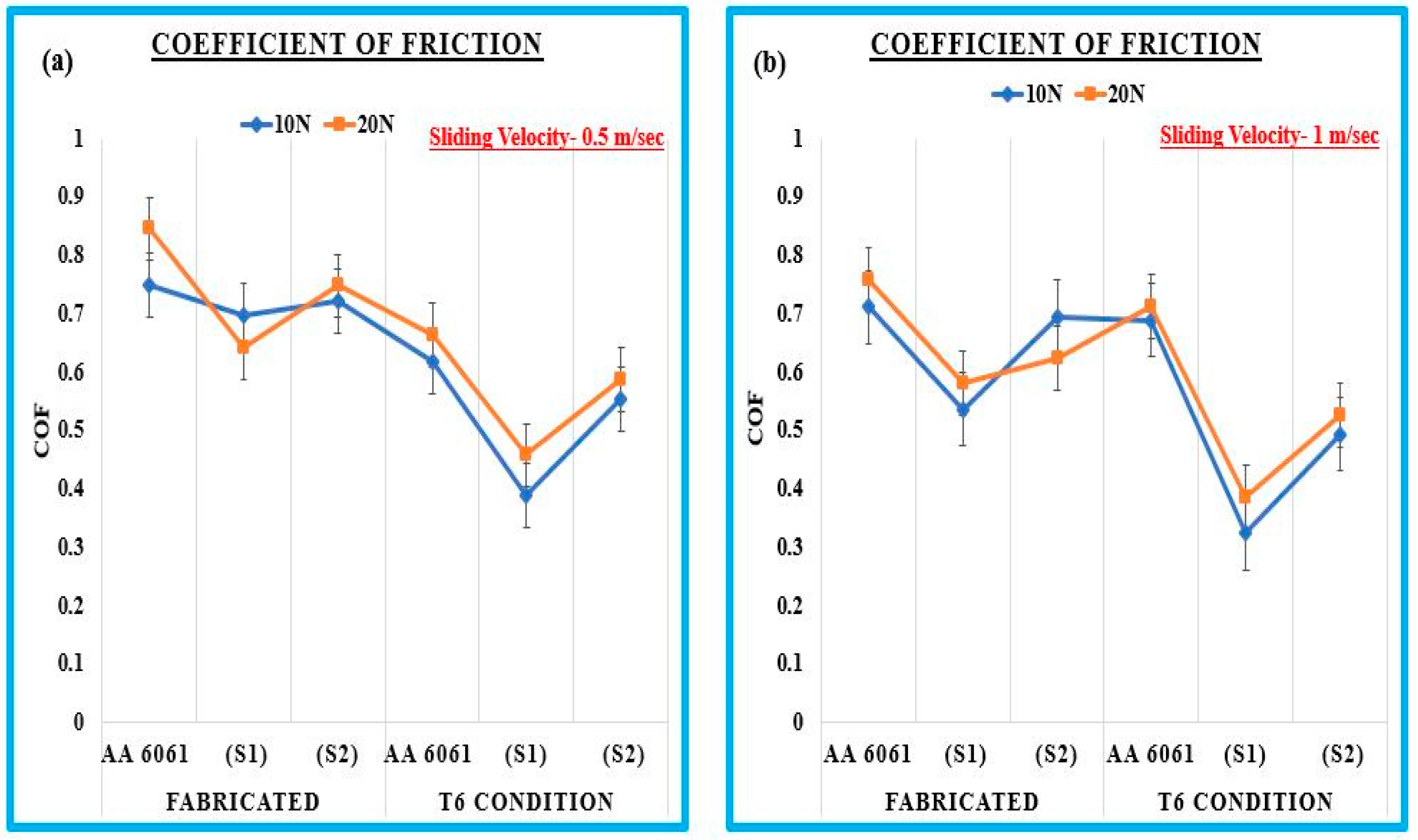
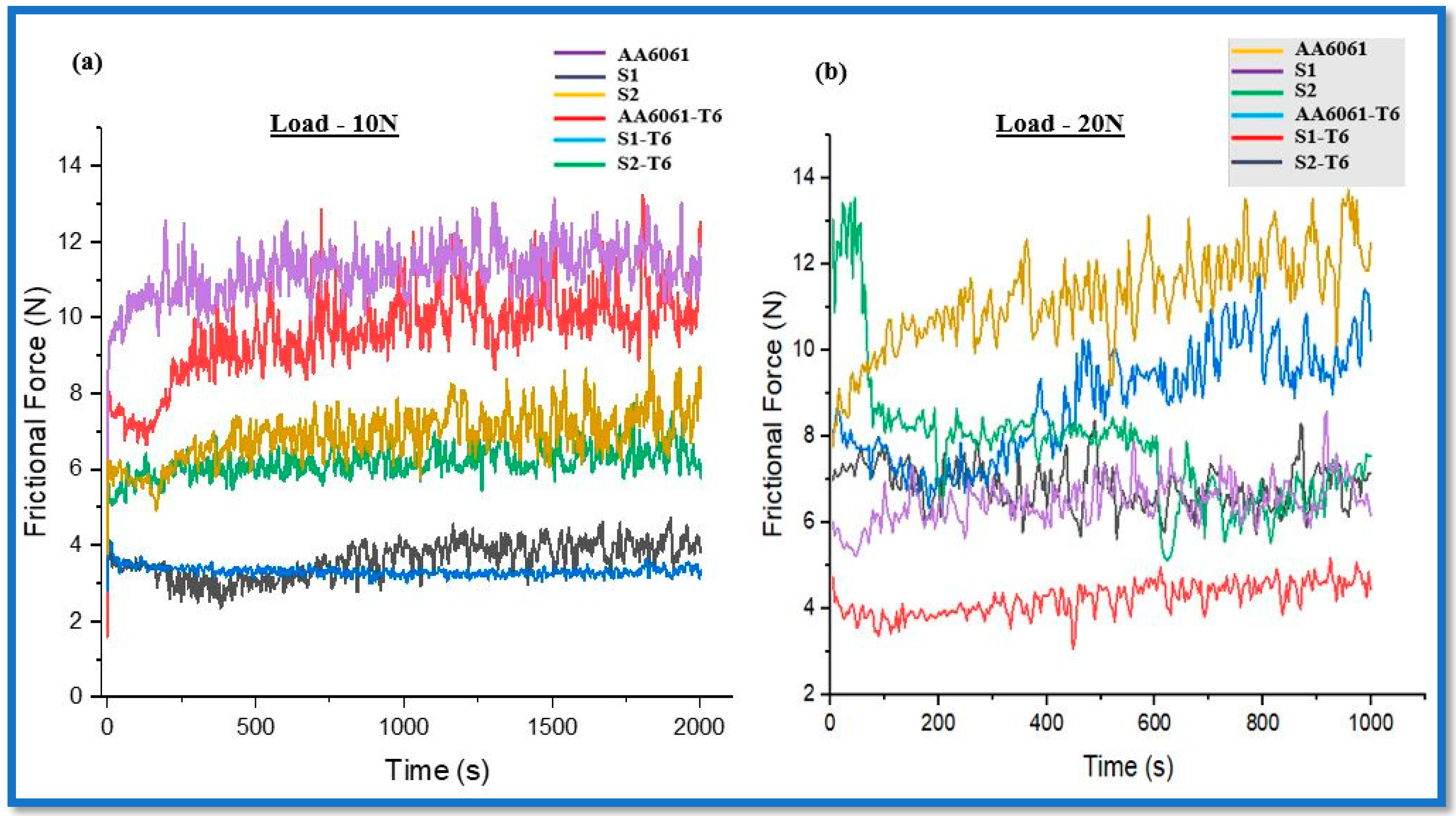
3.1.3. Effect on Coefficient of Friction
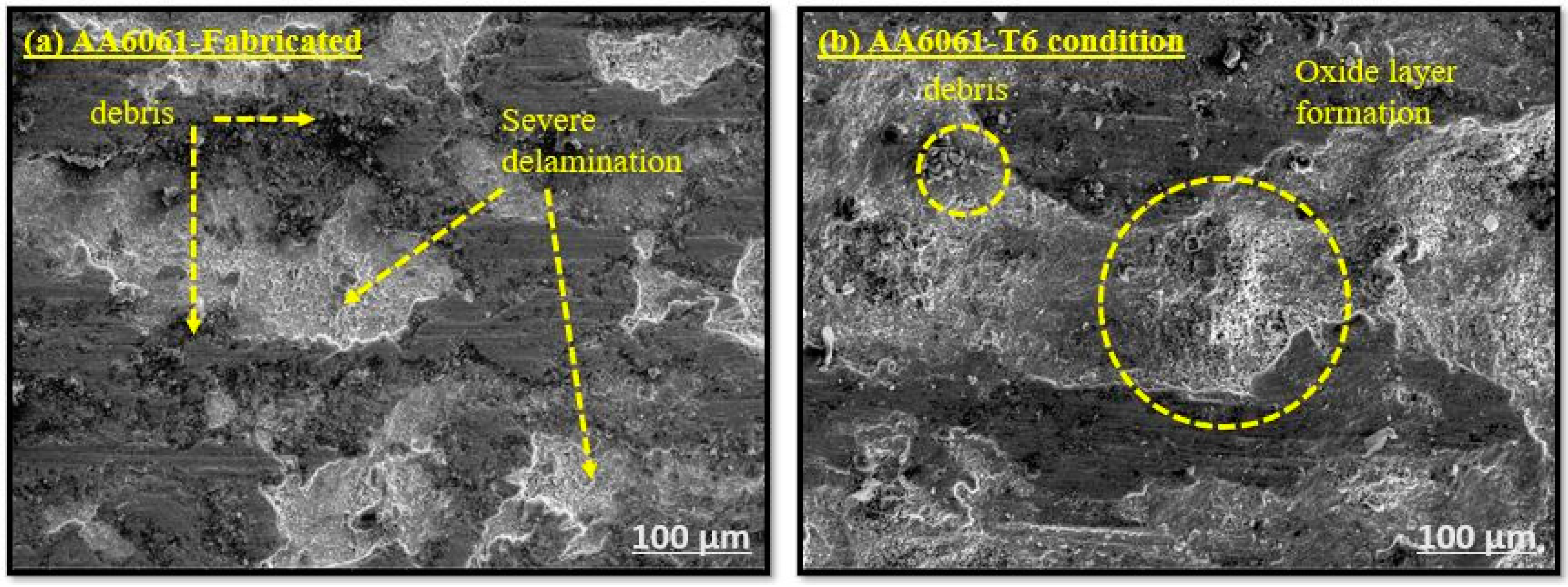
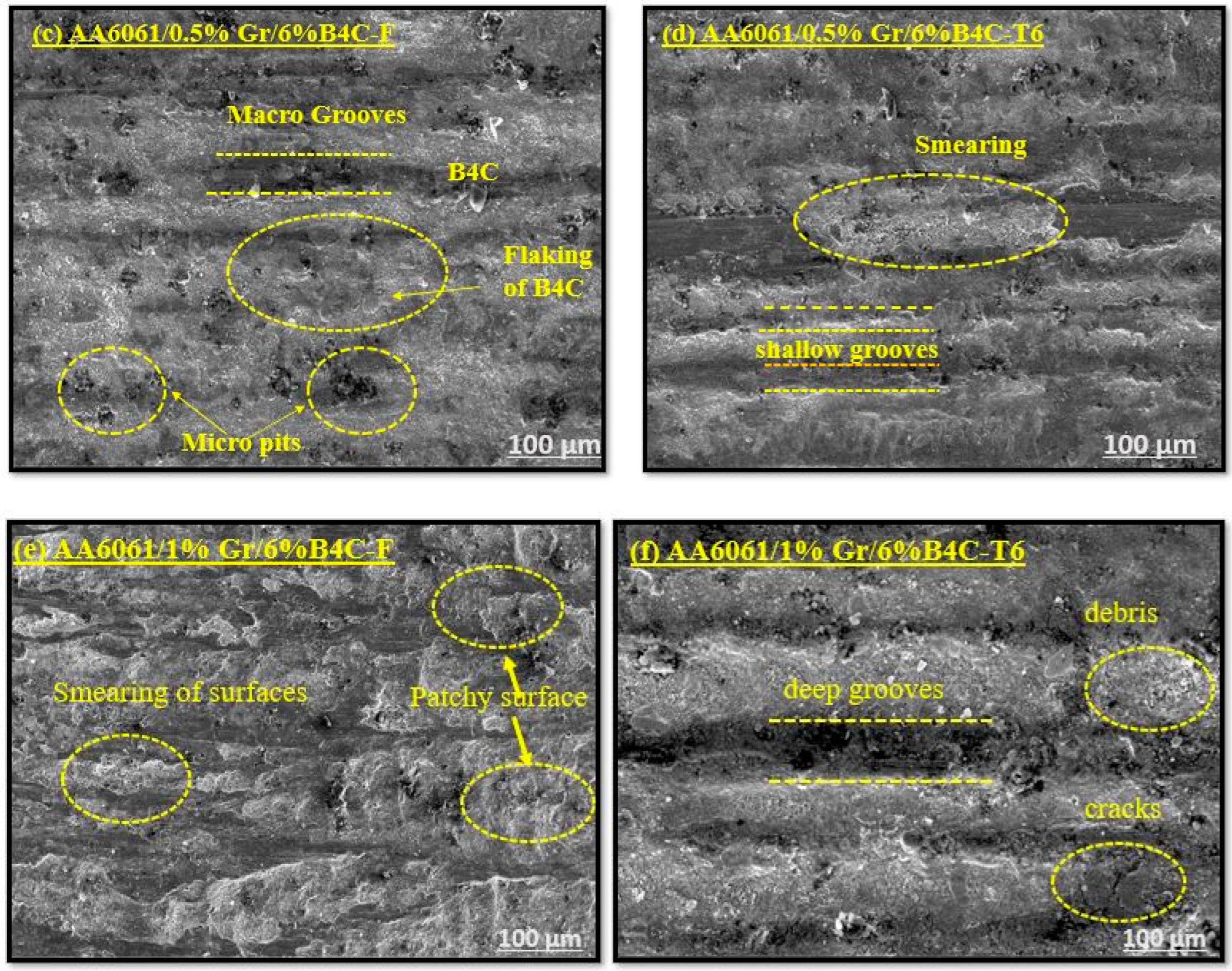
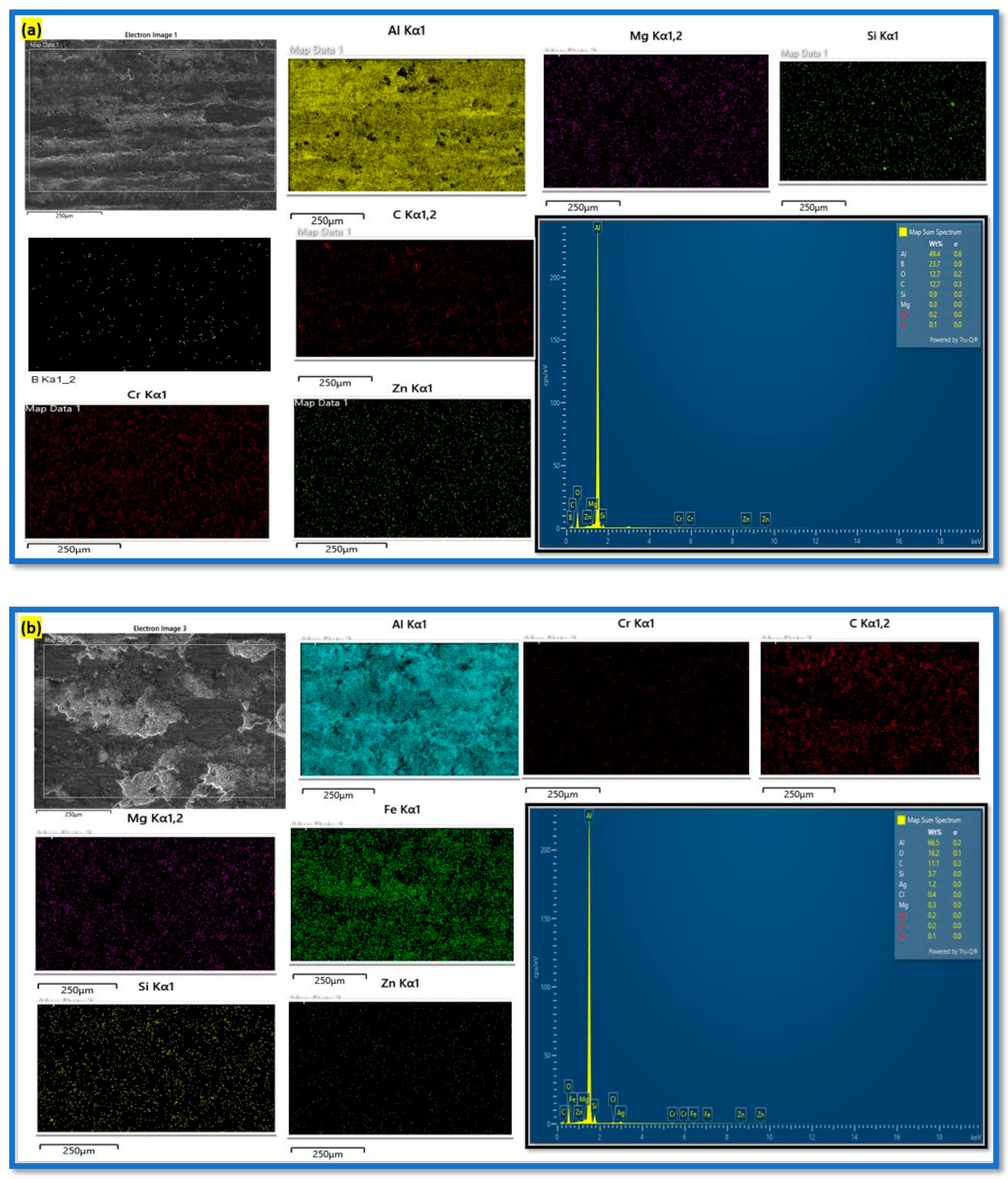
3.1.4. Worn Surface Analysis
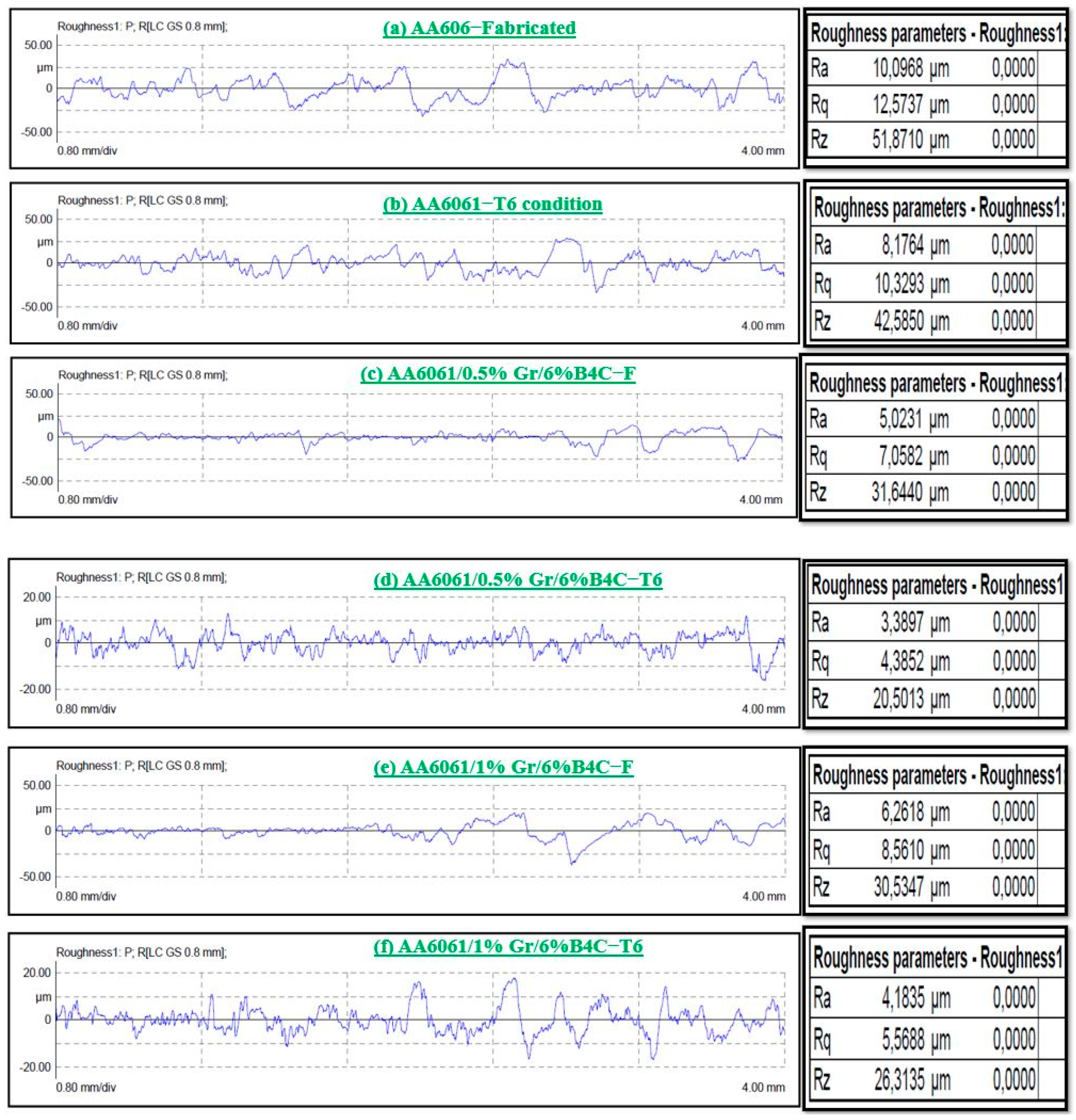
3.1.5. Surface Roughness Analysis
3.2. Corrosion Behaviour
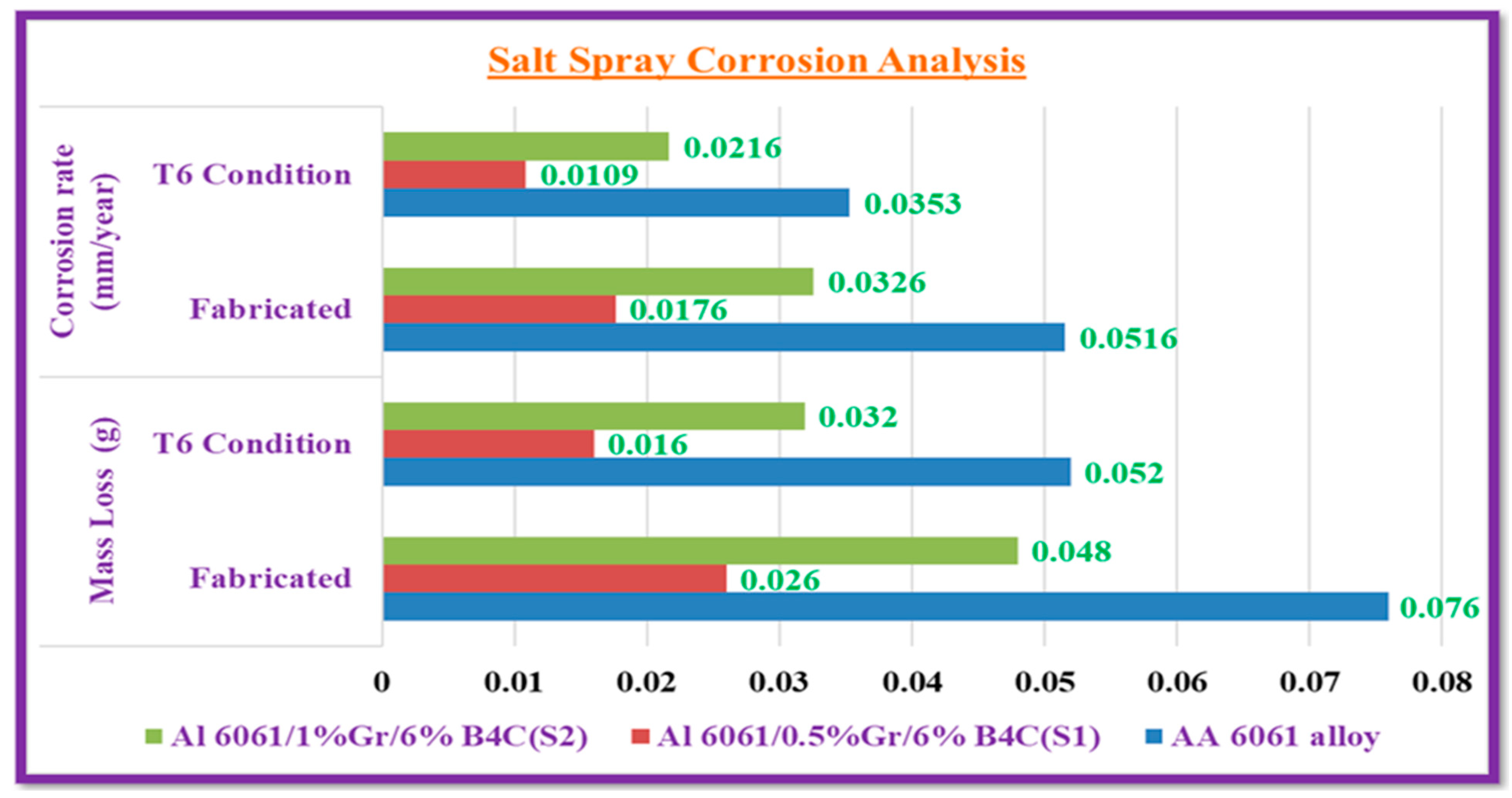
3.2.1. Salt Spray Corrosion
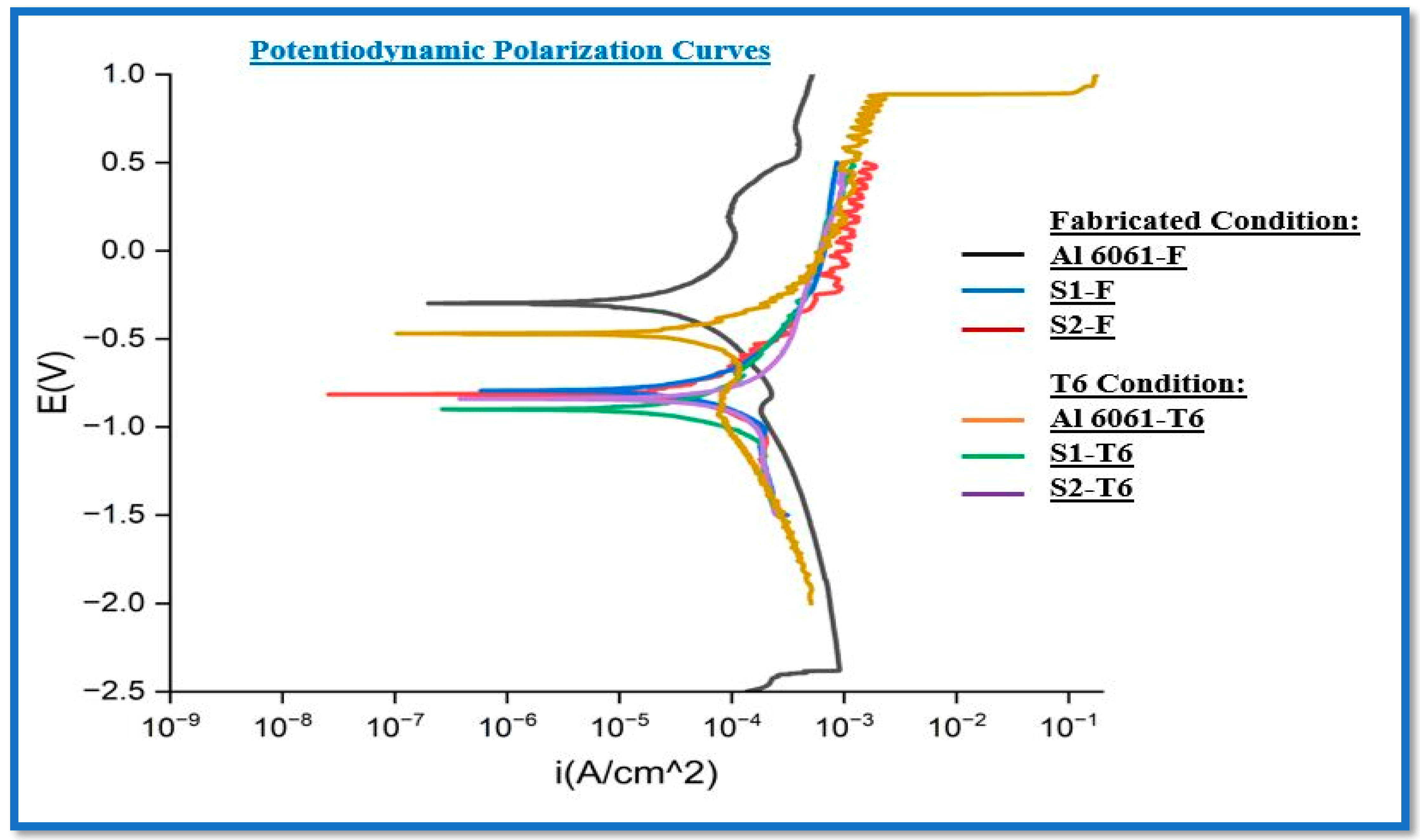
3.2.2. Electrochemical Corrosion
4. Conclusions
- ❖
- The Al6061-0.5% Gr-6% B4C (S1) exhibits good wear resistance after the T6 heat treatment condition compared to the fabricated samples. This sample shows less wear loss, lower mass loss, and reduced COF value at a load of 10 N, 20 N, and the sliding velocity of 0.5 and 1 m/s.
- ❖
- The synergistic effect of graphene’s lubricating property and B4C’s load-bearing capacity helps to resist wear loss by forming the oxide layer on the composite surface. The worn surface SEM microstructure reveals the change in wear from abrasive to adhesive wear at a higher load and sliding velocity.
- ❖
- The steady state wear condition was observed in the graphene and B4C reinforced AA6061 alloy. The SEM images of the worn surface show the microcracks, delamination of layers, and deep grooves. The sample AA6061/0.5%Gr/6%B4C exhibits a lower surface roughness value after the T6 condition. The Ra value is 3.389 µm and Rz is 20.513 µm.
- ❖
- Al6061/0.5% Gr/6% B4C (S1) has a low corrosion rate of 0.0109 mm/year when heat-treated T6 and 0.0176 mm/year when fabricated. The weight reduction is 0.016 g for T6 and 0.026 g for fabricated. In the acidic environment, refined grains, secondary phases, and the formation of an oxide layer on the surface decrease corrosion after the T6 condition.
- ❖
- In the as-fabricated (F) condition, the AA6061/0.5%Gr/6%B4C composite demonstrates an Ecorr of −0.789 and Icorr of 3.592 µA/cm2 and a corrosion rate of 0.039 mm/year. Transitioning to the T6 condition further decreases Icorr to 2.514 µA/cm2, Ecorr to −0.814, and the corrosion rate to 0.027 mm/year.
- ❖
- The results show that an increase in the addition of graphene wt.% from 0.5 to 1 to Al 6061 alloy matrix, deteriorated the wear and corrosive properties of the hybrid matrix composites.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uthayakumar, M.; Aravindan, S.; Rajkumar, K. Wear performance of Al–SiC–B4C hybrid composites under dry sliding conditions. Mater. Des. 2013, 47, 456–464. [Google Scholar] [CrossRef]
- Umanath, K.; Palanikumar, K.; Selvamani, S.T. Analysis of dry sliding wear behaviour of Al6061/SiC/Al2O3 hybrid metal matrix composites. Compos. Part B 2013, 53, 159–168. [Google Scholar] [CrossRef]
- Ren, Q.; Cui, G.; Li, T.; Hassani, M.; Liu, Y.; Kou, Z. High-temperature wear behavior of cobalt matrix composites reinforced by LaF3 and CeO2. Tribol. Lett. 2021, 69, 149. [Google Scholar] [CrossRef]
- Zhu, H.; Jar, C.; Song, J.; Zhao, J.; Li, J.; Xie, Z. High temperature dry sliding friction and wear behavior of aluminum matrix composites (Al3Zr + α-Al2O3)/Al. Tribol. Int. 2012, 48, 78–86. [Google Scholar] [CrossRef]
- Behnamian, Y.; Serate, D.; Aghaie, E.; Zahiri, R.; Tolentino, Z.; Niazi, H.; Mostafaei, A. Tribological behavior of ZK60 magnesium matrix composite reinforced by hybrid MWCNTs/B4C prepared by stir casting method. Tribol. Int. 2022, 165, 107299. [Google Scholar] [CrossRef]
- Zhang, W. A review of tribological properties for boron carbide ceramics. Prog. Mater. Sci. 2021, 116, 100718. [Google Scholar] [CrossRef]
- Krstić, J.; Jovanović, J.; Gajević, S.; Miladinović, S.; Vaxevanidis, N.M.; Kiss, I.; Stojanović, B. Application of Metal Matrix Nanocomposites in Engineering. Adv. Eng. Lett. 2024, 3, 180–190. [Google Scholar] [CrossRef]
- Singh, J.; Chauhan, A. Overview of aluminium matrix composites for automotive applications. Int. J. Appl. Eng. Res. 2014, 9, 959–966. [Google Scholar]
- Raj, P.; Biju, P.L.; Deepanraj, B.; Menachery, N. A systematic review on characterization of hybrid aluminium nanocomposites. Mater. Today Proc. 2023, 72, 2139–2150. [Google Scholar] [CrossRef]
- Sharath, B.N.; Madhu, K.S.; Venkatesh, C.V. Experimental Study on Dry Sliding Wear Behaviour of Al-B4C-Gr Metal Matrix Composite at Different Temperatures. Appl. Mech. Mater. 2019, 895, 96–101. [Google Scholar] [CrossRef]
- Li, J.; Zhang, X.; Geng, L. Improving graphene distribution and mechanical properties of GNP/Al composites by cold drawing. Mater. Des. 2018, 144, 159–168. [Google Scholar] [CrossRef]
- Zamani, N.A.B.N.; Iqbal, A.A.; Nuruzzaman, D.M. Tribo-mechanical Characterization of Self-Lubricating Aluminium Based Hybrid Metal Matrix Composite Fabricated via Powder Metallurgy. Materialia 2020, 14, 100936. [Google Scholar] [CrossRef]
- Ghodrati, H.; Ghomashchi, R. Effect of graphene dispersion and interfacial bonding on the mechanical properties of metal matrix composites: An overview. FlatChem 2019, 16, 100113. [Google Scholar] [CrossRef]
- Şenel, M.C.; Gürbüz, M.; Koç, E. Fabrication and characterization of synergistic Al-SiC-GNPs hybrid composites. Compos. Part B Eng. 2018, 154, 1–9. [Google Scholar] [CrossRef]
- Zeng, X.; Yu, J.; Fu, D.; Zhang, H.; Teng, J. Wear characteristics of hybrid aluminum-matrix composites reinforced with well-dispersed reduced graphene oxide nanosheets and silicon carbide particulates. Vacuum 2018, 155, 364–375. [Google Scholar] [CrossRef]
- Tenali, N.; Ganesan, G.; Babu, P.R. An Investigation on the Mechanical and Tribological Properties of an Ultrasonic-Assisted Stir Casting Al-Cu-Mg Matrix-Based Composite Reinforced with Agro Waste Ash Particles. Appl. Eng. Lett. 2024, 9, 46–63. [Google Scholar] [CrossRef]
- Srikanth, K.M.; Sagar, S.R.; Jayasimha, R. Mechanical and Tribological Properties of Al 6061 Alloy Reinforced with Gr–WC Particulates Using Powder Metallurgy Technique. IOP Conf. Series Mat. Sci. Eng. 2021, 1059, 012030. [Google Scholar] [CrossRef]
- Çelebi, M.; Çanakçı, A.; Güler, O.; Karabacak, H.; Akgül, B.; Özkaya, S. A study on the improvement of wear and corrosion properties of ZA40/Graphene/B4C hybrid nanocomposites. J. Alloys Compd. 2023, 966, 171628. [Google Scholar] [CrossRef]
- Martin, S.; Kandemir, S.; Antonov, M. Investigation of the high temperature dry sliding wear behavior of graphene nanoplatelets reinforced aluminum matrix composites. J. Compos. Mater. 2021, 55, 1769–1782. [Google Scholar] [CrossRef]
- Dursun, T.; Soutis, C. Recent developments in advanced aircraft aluminium alloys. Mater. Des. 2014, 56, 862–871. [Google Scholar] [CrossRef]
- Bharathiraja, P.; Anthony Xavior, M. Effect of B4C and graphene on the microstructural and mechanical properties of Al6061 matrix composites. J. Mater. Res. Technol. 2024, 31, 496–505. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Luo, Y.; Gao, Z.; Li, Y.; Zhao, Y.; Liao, Y.; Wu, C.; Jin, M. Influence of synergistic strengthening effect of B4C and TiC on tribological behaviour of copper-based powder metallurgy. Ceram. Int. 2022, 49, 2978–2990. [Google Scholar] [CrossRef]
- Gajević, S.; Miladinović, S.; Güler, O.; Özkaya, S.; Stojanović, B. Optimization of Dry Sliding Wear in Hot-Pressed Al/B4C Metal Matrix Composites Using Taguchi Method and ANN. Materials 2024, 17, 4056. [Google Scholar] [CrossRef]
- Villanueva, E.; Albizuri, J.; Caballero, P.; Guraya, T.; Vicario, I. Prediction of Wear Rate by a New Direct Method Using the Friction Coefficient Curve. J. Manuf. Mater. Process. 2025, 9, 6. [Google Scholar] [CrossRef]
- Li, Y.Z.; Wang, Q.Z.; Wang, W.G.; Xiao, B.L.; Ma, Z.Y. Effect of interfacial reaction on age-hardening ability of B4C/6061Al composites. Mater. Sci. Eng. A 2015, 620, 445–453. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, H.; Dong, X.; Ji, S. The effects of varying Mg and Si levels on the microstructural inhomogeneity and eutectic Mg2Si morphology in die-cast Al–Mg–Si alloys. J. Mater. Sci. 2019, 54, 5773–5787. [Google Scholar] [CrossRef]
- Kumar, P.L.; Lombardi, A.; Byczynski, G.; Murty, S.V.S.N.; Murty, B.S.; Bichler, L. Recent advances in aluminium matrix composites reinforced with graphene-based nanomaterial: A critical review. Prog. Mater. Sci. 2022, 128, 100948. [Google Scholar] [CrossRef]
- Liu, T.; Lyu, W.; Li, Z.; Wang, S.; Wang, X.; Jiang, J.; Jiang, X. Recent progress on corrosion mechanisms of graphene-reinforced metal matrix composites. Nanotechnol. Rev. 2023, 12, 20220566. [Google Scholar] [CrossRef]
- Fabrizi, A.; Capuzzi, S.; De Mori, A.; Timelli, G. Effect of T6 Heat Treatment on the Microstructure and Hardness of Secondary AlSi9Cu3(Fe) Alloys Produced by Semi-Solid SEED Process. Metals 2018, 8, 750. [Google Scholar] [CrossRef]
- Barros, A.; Cruz, C.; Garcia, A.; Cheung, N. Corrosion behavior of an Al–Sn–Zn alloy: Effects of solidification microstructure characteristics. J. Mater. Res. Technol. 2021, 12, 257–263. [Google Scholar] [CrossRef]
- Akçamlı, N.; Şenyurt, B.; Gökçe, H.; Ağaoğulları, D. Powder metallurgical fabrication of graphene reinforced near-eutectic Al-Si matrix composites: Microstructural, mechanical and electrochemical characterization. Eng. Sci. Technol. Int. J. 2022, 31, 101052. [Google Scholar] [CrossRef]

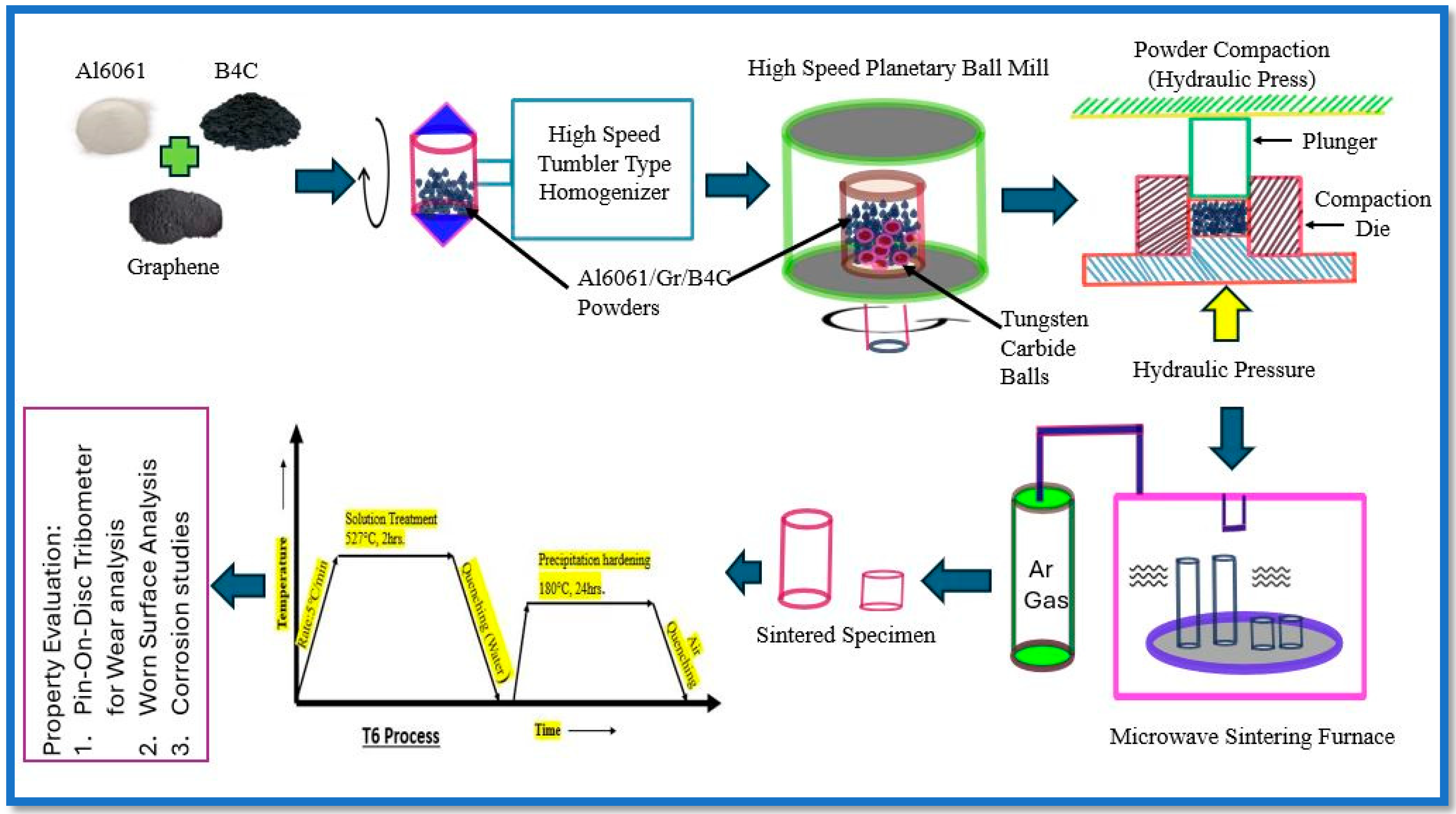


| S No. | Sample Description | Graphene (wt.%) | B4C (wt.%) | Sample Conditions |
|---|---|---|---|---|
| 1 | S1-(Al6061-0.5%Gr-6% B4C) | 0.5 | 6 | 1. As-Fabricated (F) 2. T6 temper (T6) |
| 2 | S2-(Al6061-1%Gr-6% B4C) | 1 | 6 | |
| 3 | Al6061 alloy | - | - |
| S. No: | Sample Description | Hardness (HV) |
|---|---|---|
| 1. | AA 6061-F | 54 |
| 2. | AA 6061-T6 | 61 |
| 3. | AA6061/0.5% Gr/6%B4C-F | 82 |
| 4. | AA6061/0.5% Gr/6%B4C-T6 | 91 |
| 5. | AA6061/1% Gr/6%B4C-F | 74 |
| 6. | AA6061/1% Gr/6%B4C-T6 | 83 |
| S. No | Sample Description | Ra (μm) | Rq (μm) | Rz (μm) |
|---|---|---|---|---|
| 1. | AA 6061-F | 10.096 | 12.577 | 51.872 |
| 2. | AA 6061-T6 | 8.174 | 10.329 | 42.587 |
| 3. | Al6061-0.5%Gr-6% B4C-F | 5.023 | 7.058 | 31.644 |
| 4. | Al6061-0.5%Gr-6% B4C-T6 | 3.389 | 4.385 | 20.513 |
| 5. | Al6061-1%Gr-6% B4C-F | 6.261 | 8.568 | 30.534 |
| 6. | Al6061-1%Gr-6% B4C-T6 | 4.183 | 5.568 | 26.313 |
| S. No | Sample Description | Ecorr (V) | Icorr (μA/cm2) | Corrosion Rate | |
|---|---|---|---|---|---|
| (mm/year) | (mpy) | ||||
| 1. | AA 6061-F | −0.472 | 6.842 | 0.075 | 2.952 |
| 2. | AA 6061-T6 | −0.357 | 4.864 | 0.053 | 2.086 |
| 3. | Al6061-0.5%Gr-6% B4C-F | −0.789 | 3.592 | 0.039 | 1.532 |
| 4. | Al6061-0.5%Gr-6% B4C-T6 | −0.814 | 2.514 | 0.027 | 1.062 |
| 5. | Al6061-1%Gr-6% B4C-F | −0.842 | 8.762 | 0.096 | 3.779 |
| 6. | Al6061-1%Gr-6% B4C-T6 | −0.896 | 4.213 | 0.045 | 1.836 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parasuraman, B.; Michael, A.X. Effect of T6 Tempering on the Wear and Corrosive Properties of Graphene and B4C Reinforced Al6061 Matrix Composites. J. Manuf. Mater. Process. 2025, 9, 82. https://doi.org/10.3390/jmmp9030082
Parasuraman B, Michael AX. Effect of T6 Tempering on the Wear and Corrosive Properties of Graphene and B4C Reinforced Al6061 Matrix Composites. Journal of Manufacturing and Materials Processing. 2025; 9(3):82. https://doi.org/10.3390/jmmp9030082
Chicago/Turabian StyleParasuraman, Bharathiraja, and Anthony Xavior Michael. 2025. "Effect of T6 Tempering on the Wear and Corrosive Properties of Graphene and B4C Reinforced Al6061 Matrix Composites" Journal of Manufacturing and Materials Processing 9, no. 3: 82. https://doi.org/10.3390/jmmp9030082
APA StyleParasuraman, B., & Michael, A. X. (2025). Effect of T6 Tempering on the Wear and Corrosive Properties of Graphene and B4C Reinforced Al6061 Matrix Composites. Journal of Manufacturing and Materials Processing, 9(3), 82. https://doi.org/10.3390/jmmp9030082







