Effect of Powder Preparation of FeNiCoCrMo0.5Al1.3 High-Entropy Alloy on the Phase Composition and Properties of High-Velocity Oxy-Fuel-Sprayed Coatings
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
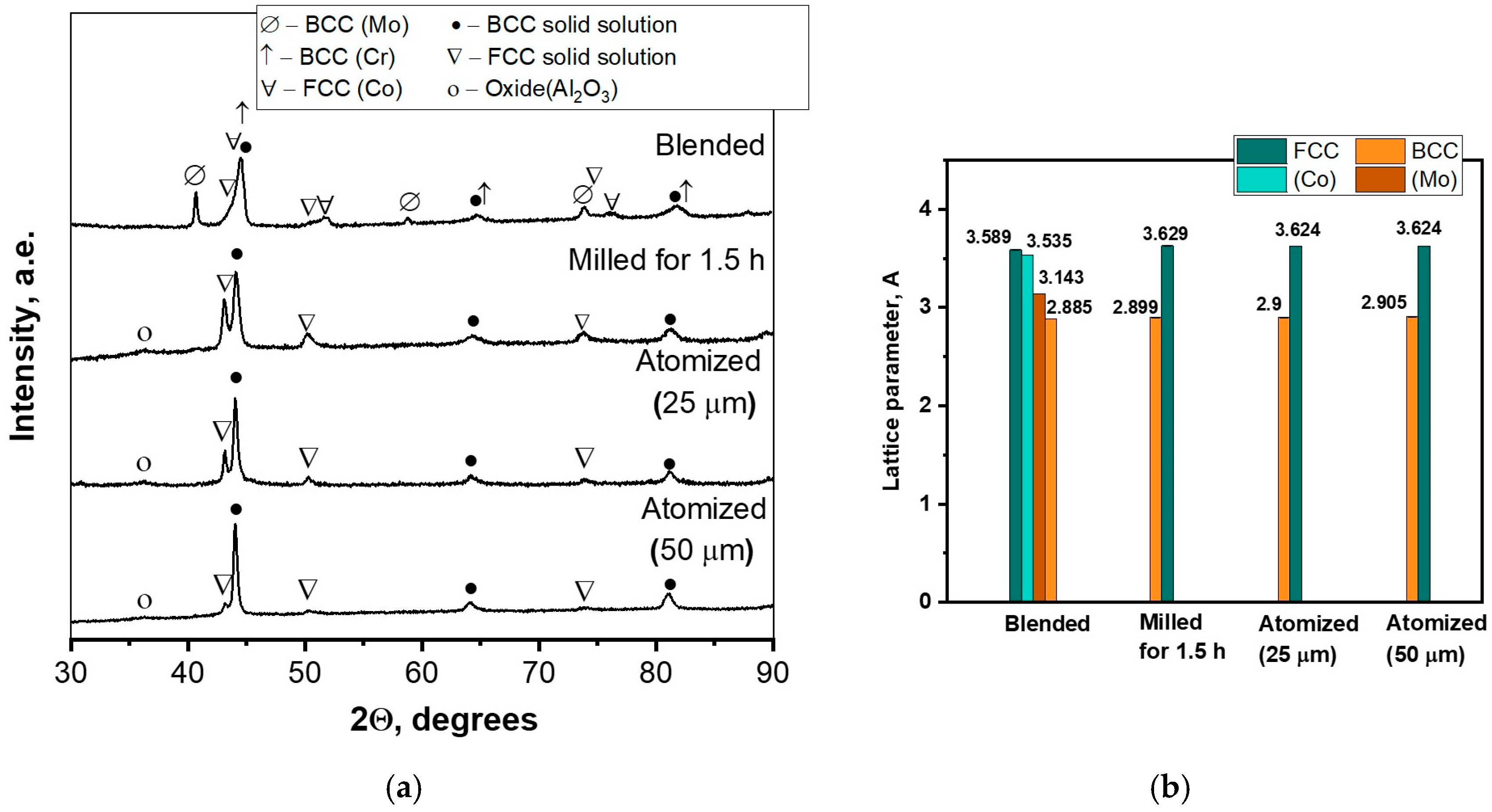
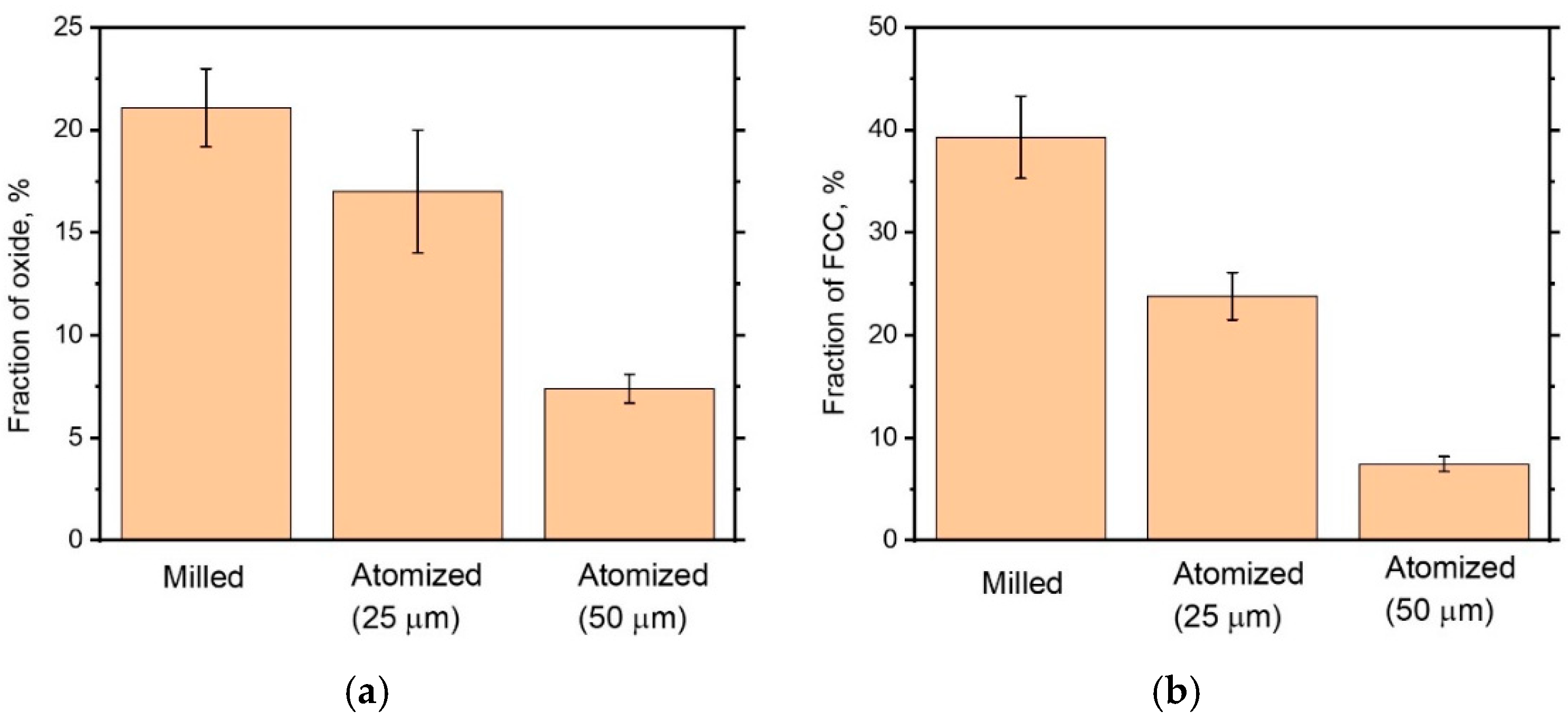
3.1. Powder Characterization
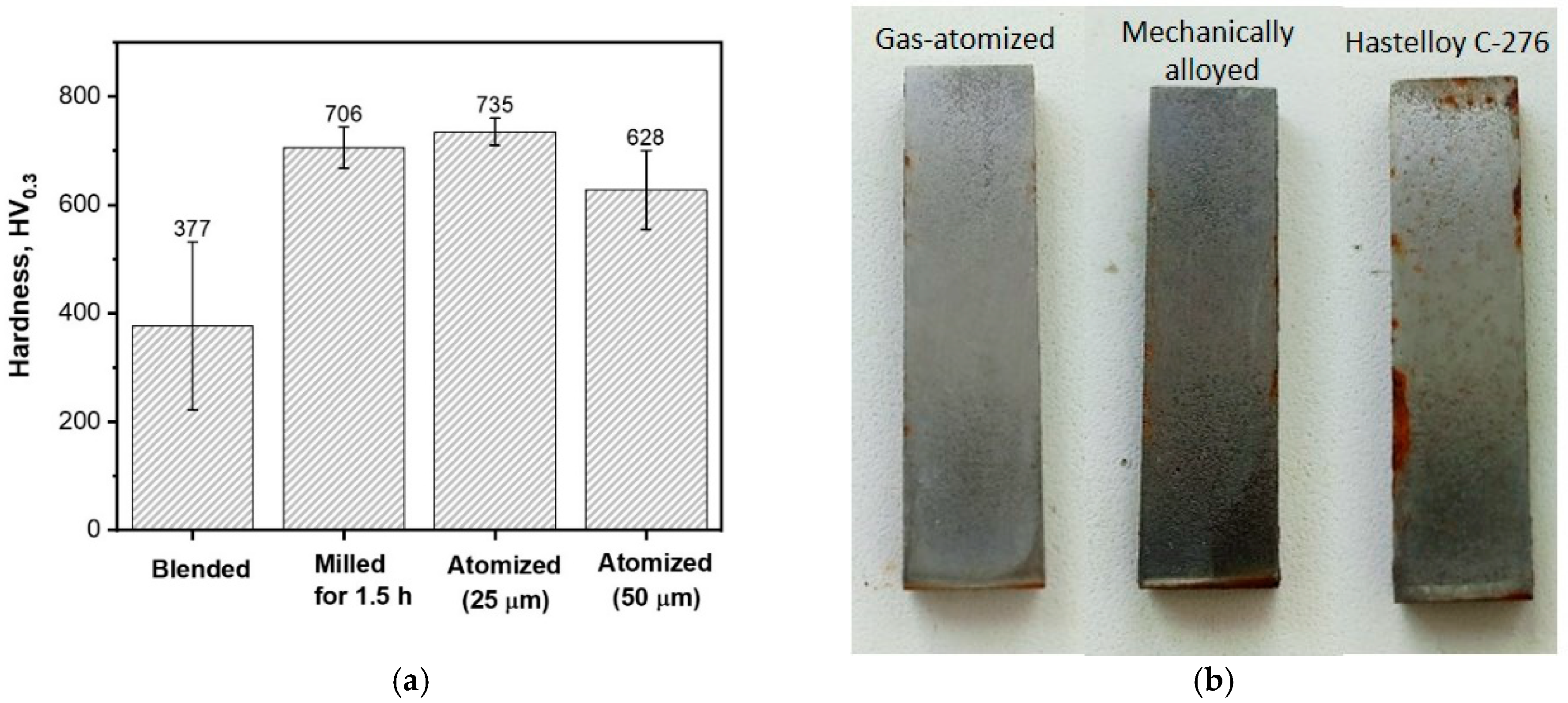
3.2. Coating Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Multicomponent Alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A Critical Review of High Entropy Alloys and Related Concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of High-Entropy Stabilized Solid-Solution in Multi-Component Alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Sharma, P.; Dwivedi, V.K.; Dwivedi, S.P. Development of High Entropy Alloys: A Review. Mater. Today Proc. 2020, 43, 502–509. [Google Scholar] [CrossRef]
- Rogachev, A.S. Structure, Stability, and Properties of High-Entropy Alloys. Phys. Met. Metallogr. 2020, 121, 733–764. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, B.; Liu, B.; Cao, Y.; Li, J.; Fang, Q.; Liaw, P.K. A Focused Review on Engineering Application of Multi-Principal Element Alloy. Front. Mater. 2022, 8, 816309. [Google Scholar] [CrossRef]
- Han, L.; Zhu, S.; Rao, Z.; Scheu, C.; Ponge, D.; Ludwig, A.; Zhang, H.; Gutfleisch, O.; Hahn, H.; Li, Z.; et al. Multifunctional high-entropy materials. Nat. Rev. Mater. 2024, 30, 1–20. [Google Scholar] [CrossRef]
- Wu, S.; Wang, G.; Wang, Q.; Jia, Y.; Yi, J.; Zhai, Q.; Liu, J.; Sun, B.; Chu, H.; Shen, J.; et al. Enhancement of strength-ductility trade-off in a high-entropy alloy through a heterogeneous structure. Acta Mater. 2019, 165, 444–458. [Google Scholar] [CrossRef]
- Gao, X.; Lu, Y.; Zhang, B.; Liang, N.; Wu, G.; Sha, G.; Liu, J.; Zhao, Y. Microstructural origins of high strength and high ductility in an AlCoCrFeNi2.1 eutectic high-entropy alloy. Acta Mater. 2017, 141, 59–66. [Google Scholar] [CrossRef]
- Wu, P.; Gan, K.; Yan, D.; Fu, Z.; Li, Z. A non-equiatomic FeNiCoCr high-entropy alloy with excellent anti-corrosion performance and strength-ductility synergy. Corros. Sci. 2021, 183, 109341. [Google Scholar] [CrossRef]
- Shi, Z.; Fang, Q.; Liaw, P.; Li, J. Corrosion-Resistant Biomedical High-Entropy Alloys: A Review. Adv. Eng. Mater. 2023, 25, 2300968. [Google Scholar] [CrossRef]
- Birbilis, N.; Choudhary, S.; Scully, J.R.; Taheri, M.L. A Perspective on Corrosion of Multi-Principal Element Alloys. Npj Mater. Degrad. 2021, 5, 2300968. [Google Scholar] [CrossRef]
- Lin, C.; Yao, Y. Corrosion-Resistant Coating Based on High-Entropy Alloys. Metals 2023, 13, 205. [Google Scholar] [CrossRef]
- Kong, D.; Guo, J.; Liu, R.; Zhang, X.; Song, Y.; Li, Z.; Guo, F.; Xing, X.; Xu, Y.; Wang, W. Effect of Remelting and Annealing on the Wear Resistance of AlCoCrFeNiTi0.5 High Entropy Alloys. Intermetallics 2019, 114, 106560. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, J.; Manladan, S.M.; Geng, K.; Hu, S. Wear Resistance of FeCoCrNiMnAlx High-Entropy Alloy Coatings at High Temperature. Appl. Surf. Sci. 2020, 512, 145736. [Google Scholar] [CrossRef]
- Feng, C.; Wang, X.; Yang, L.; Guo, Y.; Wang, Y. High Hardness and Wear Resistance in AlCrFeNiV High-Entropy Alloy Induced by Dual-Phase Body-Centered Cubic Coupling Effects. Materials 2022, 15, 6896. [Google Scholar] [CrossRef]
- Lu, J.; Li, L.; Chen, Y.; Liu, X.; Zhao, X.; Guo, F.; Xiao, P. Y-Hf Co-Doped AlCoCrFeNi High-Entropy Alloy Coating with Superior Oxidation and Spallation Resistance at 1100 °C. Corros. Sci. 2021, 182, 109267. [Google Scholar] [CrossRef]
- Shajahan, S.; Kumar, A.; Chopkar, M.; Basu, A. Oxidation Study of CoCrCuFeNiSix High Entropy Alloys. Mater. Res. Express 2019, 7, 16532. [Google Scholar] [CrossRef]
- Meghwal, A.; Anupam, A.; Murty, B.S.; Berndt, C.C.; Kottada, R.S.; Ang, A.S.M. Thermal Spray High-Entropy Alloy Coatings: A Review. J. Therm. Spray Technol. 2020, 29, 857–893. [Google Scholar] [CrossRef]
- Tejero-Martin, D.; Rezvani Rad, M.; McDonald, A.; Hussain, T. Beyond Traditional Coatings: A Review on Thermal-Sprayed Functional and Smart Coatings. J. Therm. Spray Technol. 2019, 28, 598–644. [Google Scholar] [CrossRef]
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On Coating Techniques for Surface Protection: A Review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Łatka, L.; Pawłowski, L.; Winnicki, M.; Sokołowski, P.; Małachowska, A.; Kozerski, S. Review of Functionally Graded Thermal Sprayed Coatings. Appl. Sci. 2020, 10, 5153. [Google Scholar] [CrossRef]
- Bhaskaran Nair, R.; Supekar, R.; Morteza Javid, S.; Wang, W.; Zou, Y.; McDonald, A.; Mostaghimi, J.; Stoyanov, P. High-Entropy Alloy Coatings Deposited by Thermal Spraying: A Review of Strengthening Mechanisms, Performance Assessments and Perspectives on Future Applications. Metals 2023, 13, 579. [Google Scholar] [CrossRef]
- Hsu, W.L.; Murakami, H.; Yeh, J.W.; Yeh, A.C.; Shimoda, K. On the Study of Thermal-Sprayed Ni0.2Co0.6Fe0.2CrSi0.2AlTi0.2 HEA Overlay Coating. Surf. Coat. Technol. 2017, 316, 71–74. [Google Scholar] [CrossRef]
- Li, T.; Liu, Y.; Liu, B.; Guo, W.; Xu, L. Microstructure and Wear Behavior of FeCoCrNiMo0.2 High Entropy Coatings Prepared by Air Plasma Spray and the High Velocity Oxy-Fuel Spray Processes. Coatings 2017, 7, 151. [Google Scholar]
- Rukhande, S.W.; Rathod, W.S.; Bhosale, D. High-Temperature Tribological Investigation of APS and HVOF Sprayed NiCrBSiFe Coatings on SS 316L. Tribol.-Mater. Surf. Interfaces 2022, 16, 98–109. [Google Scholar] [CrossRef]
- Rukhande, S.W.; Rathod, W.S. Tribological Behaviour of Plasma and HVOF-Sprayed NiCrSiBFe Coatings. Surf. Eng. 2020, 36, 745–755. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Lampke, T. High-Temperature Wear Behaviour of AlCoCrFeNiTi0.5 Coatings Produced by HVOF. Surf. Coat. Technol. 2020, 403, 126379. [Google Scholar] [CrossRef]
- Semikolenov, A.; Kuznetsov, P.; Bobkova, T.; Shalnova, S.; Klimova-Korsmik, O.; Klinkov, V.; Kobykhno, I.; Larionova, T.; Tolochko, O. Microstructure Evolution of FeNiCoCrAl1.3Mo0.5 High Entropy Alloy during Powder Preparation, Laser Powder Bed Fusion, and Microplasma Spraying. Materials 2021, 14, 7870. [Google Scholar] [CrossRef]
- Semikolenov, A.; Goshkoderya, M.; Uglunts, T.; Larionova, T.; Tolochko, O. Oxidation Behavior of FeNiCoCrMo0.5Al1.3 High-Entropy Alloy Powder. Materials 2024, 17, 531. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Bobzin, K.; Zhou, Z.; Zhao, L.; Öte, M.; Königstein, T.; Tan, Z.; He, D. Wear Behavior of HVOF-Sprayed Al0.6TiCrFeCoNi High Entropy Alloy Coatings at Different Temperatures. Surf. Coat. Technol. 2019, 358, 215–222. [Google Scholar] [CrossRef]
- Huang, P.K.; Yeh, J.W.; Shun, T.T.; Chen, S.K. Multi-Principal-Element Alloys with Improved Oxidation and Wear Resistance for Thermal Spray Coating. Adv. Eng. Mater. 2004, 6, 74–78. [Google Scholar] [CrossRef]
- Wang, L.M.; Chen, C.C.; Yeh, J.W.; Ke, S.T. The Microstructure and Strengthening Mechanism of Thermal Spray Coating NixCo0.6Fe0.2CrySi ZAlTi0.2 High-Entropy Alloys. Mater. Chem. Phys. 2011, 126, 880–885. [Google Scholar] [CrossRef]
- Hsu, W.L.; Yang, Y.C.; Chen, C.Y.; Yeh, J.W. Thermal Sprayed High-Entropy NiCo0.6Fe0.2Cr1.5SiAlTi0.2 Coating with Improved Mechanical Properties and Oxidation Resistance. Intermetallics 2017, 89, 105–110. [Google Scholar] [CrossRef]
- Srivastava, M.; Jadhav, M.S.; Chethan; Chakradhar, R.P.S.; Singh, S. Investigation of HVOF Sprayed Novel Al1.4Co2.1Cr0.7Ni2.45Si0.2Ti0.14 HEA Coating as Bond Coat Material in TBC System. J. Alloys Compd. 2022, 924, 166388. [Google Scholar] [CrossRef]
- Srivastava, M.; Jadhav, M.; Chethan; Chakradhar, R.P.S.; Muniprakash, M.; Singh, S. Synthesis and Properties of High Velocity Oxy-Fuel Sprayed FeCoCrNi2Al High Entropy Alloy Coating. Surf. Coat. Technol. 2019, 378, 124950. [Google Scholar] [CrossRef]
- Yin, S.; Li, W.; Song, B.; Yan, X.; Kuang, M.; Xu, Y.; Wen, K.; Lupoi, R. Deposition of FeCoNiCrMn High Entropy Alloy (HEA) Coating via Cold Spraying. J. Mater. Sci. Technol. 2019, 35, 1003–1007. [Google Scholar] [CrossRef]
- Patel, P.; Nair, R.B.; Supekar, R.; McDonald, A.; Chromik, R.R.; Moreau, C.; Stoyanov, P. Enhanced Wear Resistance of AlCoCrFeMo High Entropy Coatings (HECs) through Various Thermal Spray Techniques. Surf. Coat. Technol. 2024, 477, 130311. [Google Scholar] [CrossRef]
- Addepalli, S.N.; Joladarashi, S.; Ramesh, M.R. Elevated Temperature Tribological Performance of Non-Equiatomic CoCrNiTiWx High Entropy Alloy Coatings Developed by Mechanical Alloying and High-Velocity Oxy-Fuel Spray. Surf. Coat. Technol. 2024, 476, 130267. [Google Scholar] [CrossRef]
- Löbel, M.; Lindner, T.; Kohrt, C.; Lampke, T. Processing of AlCoCrFeNiTi High Entropy Alloy by Atmospheric Plasma Spraying. Proc. IOP Conf. Ser. Mater. Sci. Eng. Inst. Phys. Publ. 2017, 181, 012015. [Google Scholar] [CrossRef]
- Cheng, K.C.; Chen, J.H.; Stadler, S.; Chen, S.H. Properties of Atomized AlCoCrFeNi High-Entropy Alloy Powders and Their Phase-Adjustable Coatings Prepared via Plasma Spray Process. Appl. Surf. Sci. 2019, 478, 478–486. [Google Scholar] [CrossRef]
- Vaidya, M.; Muralikrishna, G.M.; Murty, B.S. High-Entropy Alloys by Mechanical Alloying: A Review. J. Mater. Res. 2019, 34, 664–686. [Google Scholar] [CrossRef]
- Torralba, J.M.; Alvaredo, P.; García-Junceda, A. High-entropy alloys fabricated via powder metallurgy. A critical review. Powder Metall. 2019, 62, 84–114. [Google Scholar] [CrossRef]
- GOST 9.308-85; Unified System of Corrosion and Ageing Protection. Metal and Non-Metal Inorganic Coatings. Methods for Accelerated Corrosion Tests. USSR State Committee for Standards: Moscow, Russia, 1985.
- Larionova, T.; Semikolenov, A.; Kuznetsov, P.; Shalnova, S.; Tolochko, O. Phase Transformation and Strengthening of the Gas-Atomized FeCoCrNiMo0.5Al1.3 Eutectic High-Entropy Alloy Powder during Annealing. Heliyon 2024, 10, 8. [Google Scholar] [CrossRef]
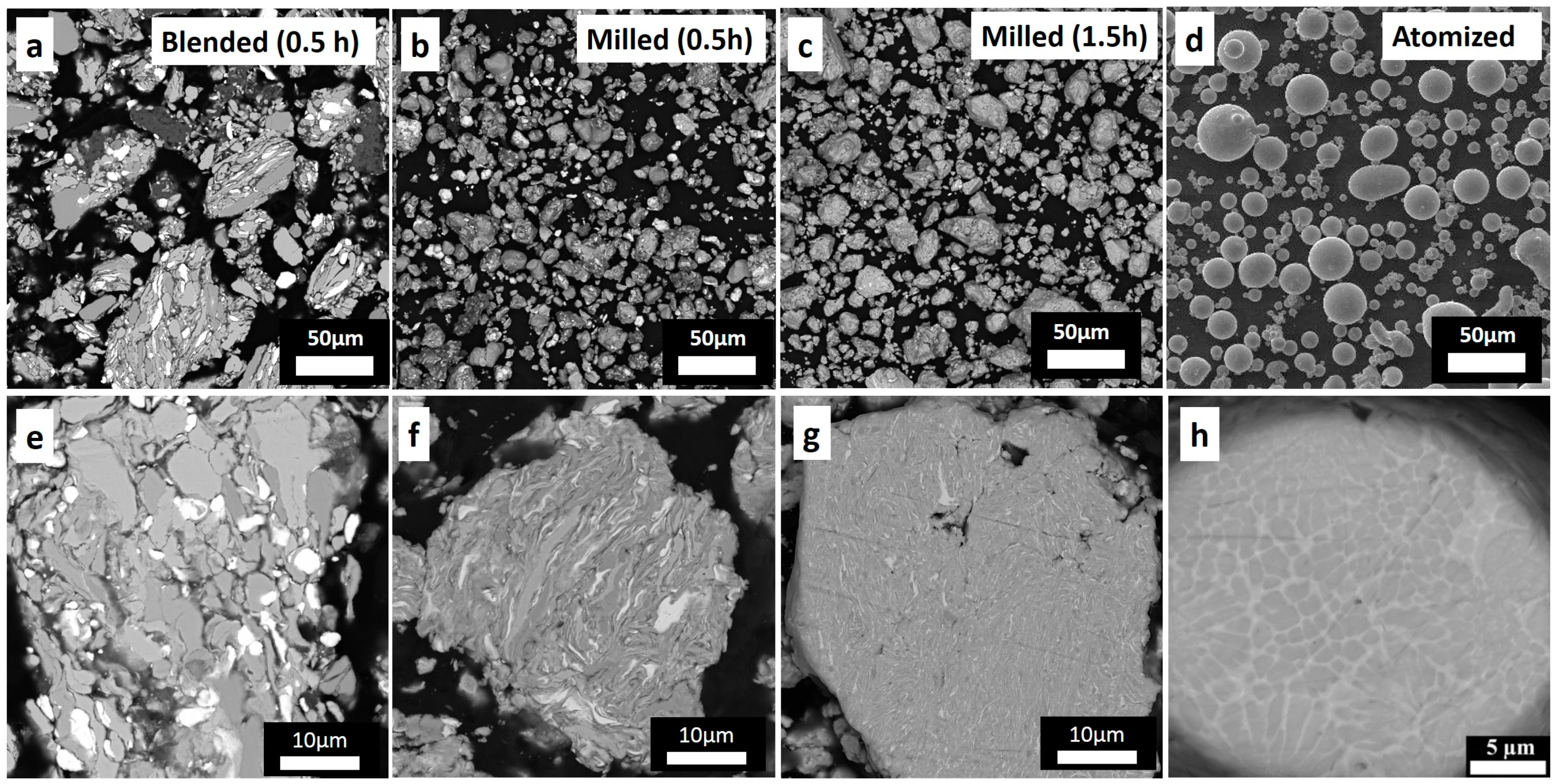
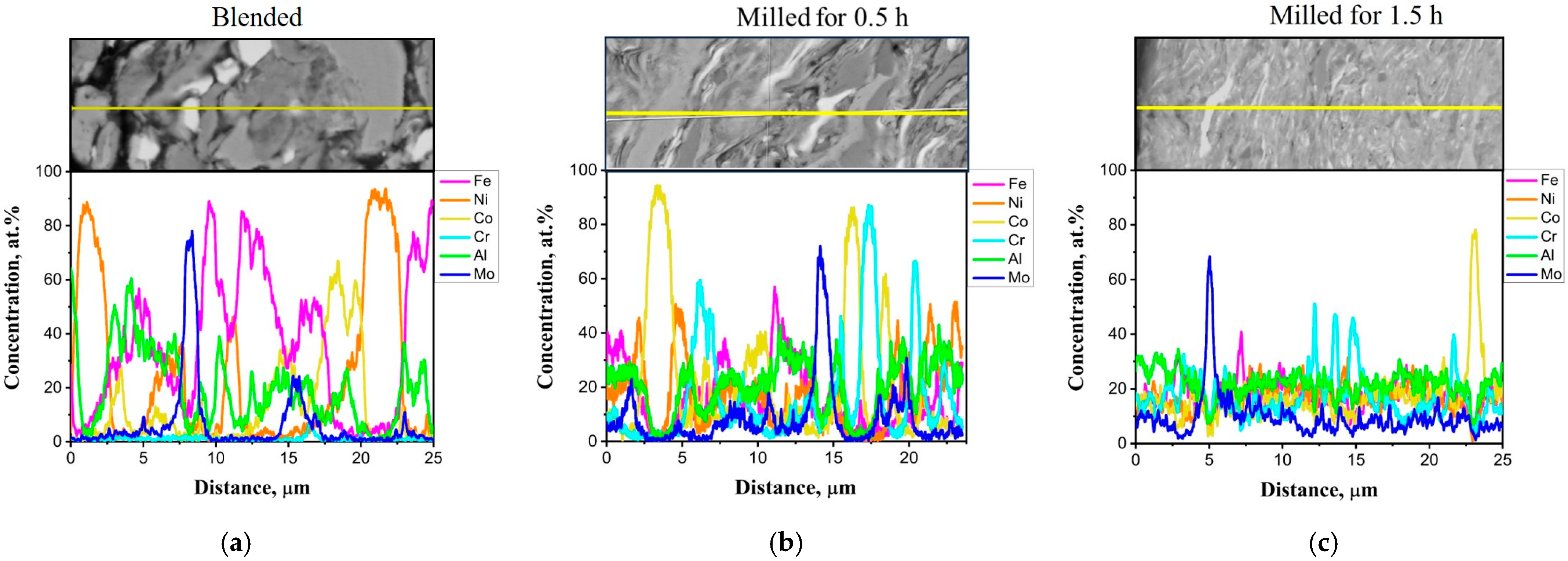
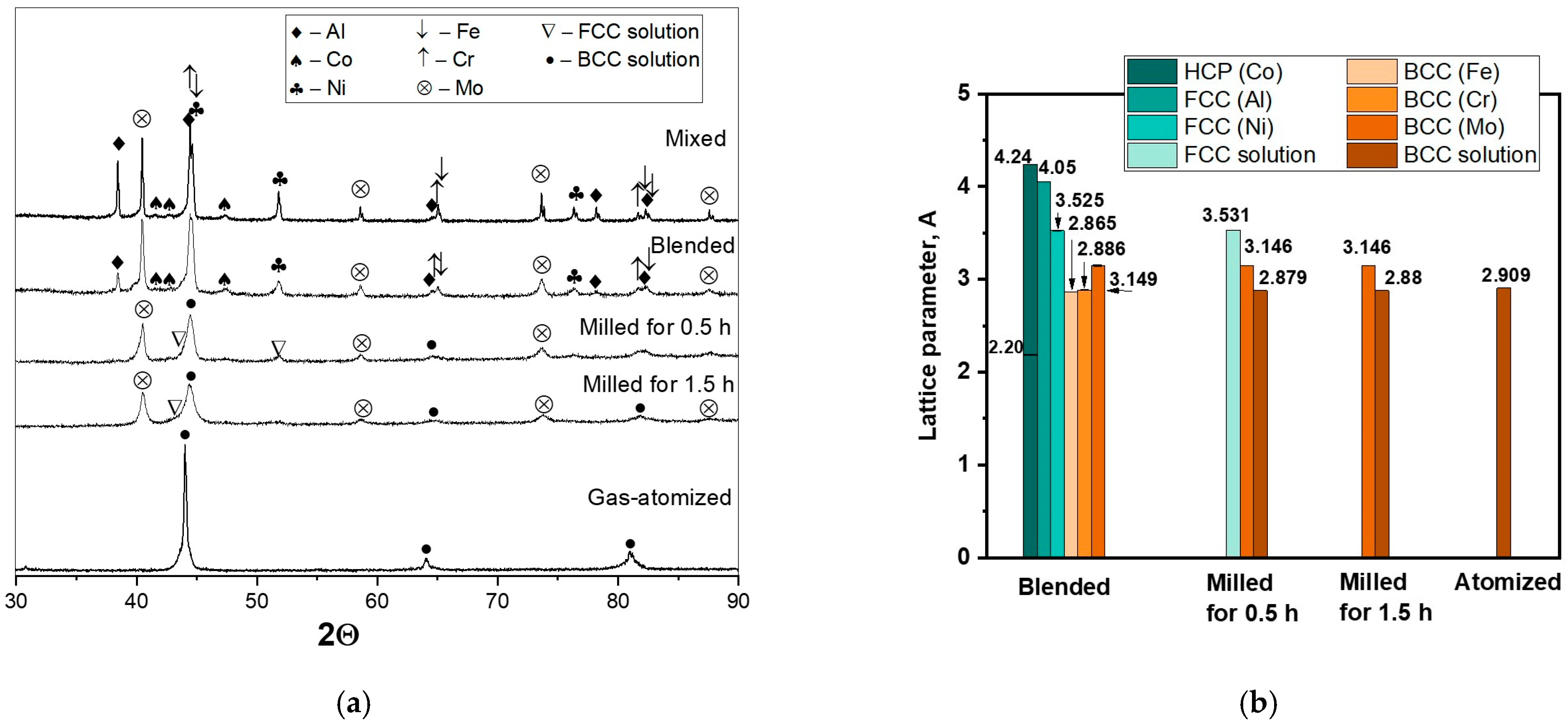

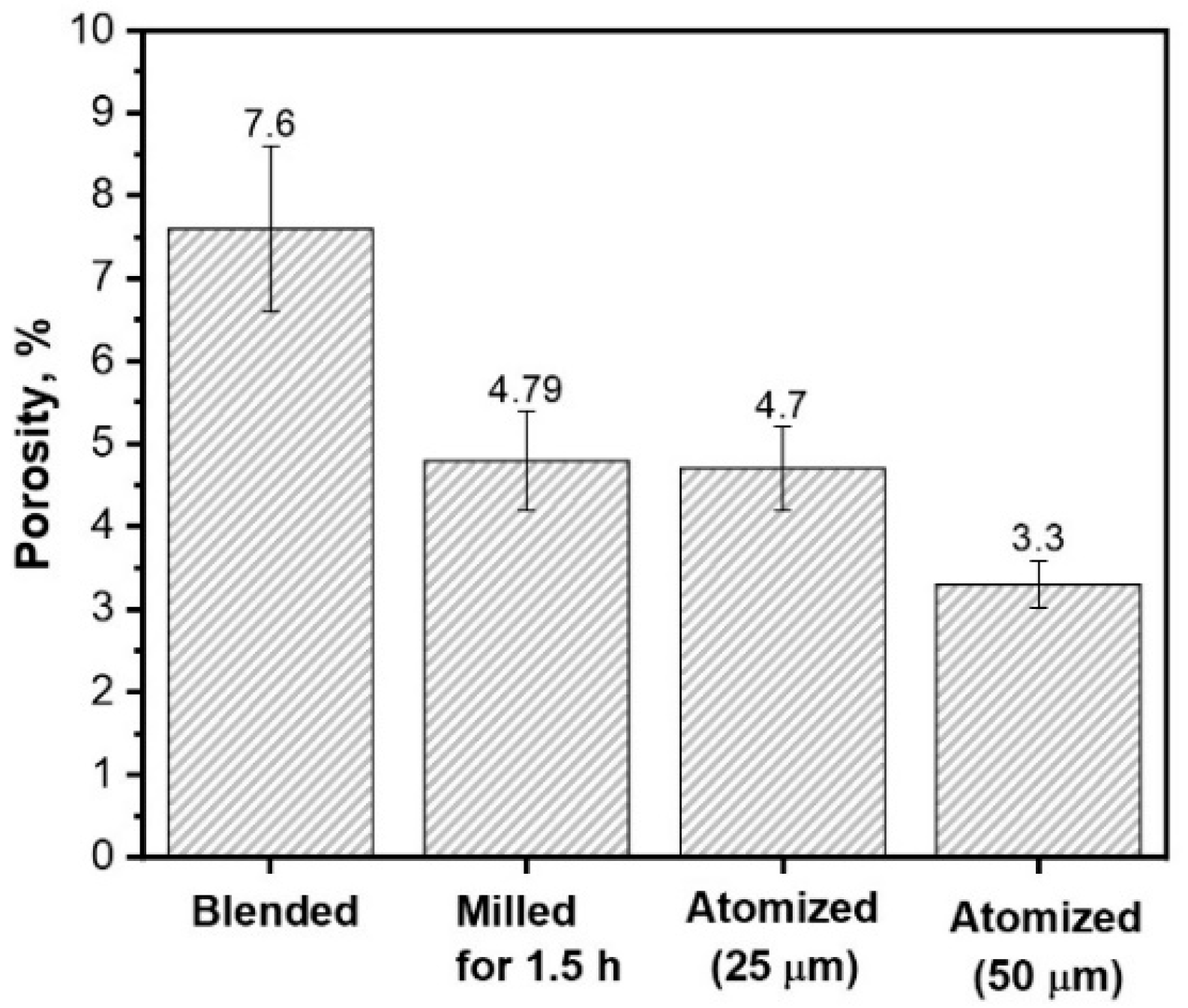

| Chemical Composition | Powder Preparation Procedure | Coating Deposition Method | Average Particle Size (µm) | Feedstock Powder Phase Composition | Coating Phase Composition | Coating Microhardness | References |
|---|---|---|---|---|---|---|---|
| AlCoCrFeNiTi | Mixing of pure metal powders and pre-alloyed FeCr13 | APS | 41.3 | BCC1 FCC(Ni) HCP(Ti) Oxide | BCC1 FCC(Ni) HCP(Ti) Oxide | 354 HV0.1 | [41] |
| AlSiTiCrFeCoNiMo0.5 | Milling of the pre-alloyed ingot | APS | - | BCC FCC1 FCC2 | BCC Oxide | 524 HV | [33] |
| Ni0.2Co0.6Fe0.2CrSi0.2AlTi0.2 | Milling of the pre-alloyed ingot | APS | 44 | BCC Cr3Si minor FCC | BCC Cr3Si Oxide | 429 HV5 | [35] |
| Al1.4Co2.1Cr0.7Ni2.45Si0.2Ti0.2 | Milling of the mechanically alloyed at 300 rpm for 5 h and then compacted bulk | HVOF | 15–20 | FCC BCC | FCC BCC Oxide | 814 HV0.1 | [36] |
| FeCoCrNi2Al | Milling of the mechanically alloyed at 300 rpm for 2 h and then compacted bulk | HVOF | 15 | FCC BCC | FCC BCC Oxide | 600 HV | [37] |
| AlCoCrFeNiTi0.5 | Gas atomization | HVOF | 26.5 | BCC | BCC Oxide | 610 HV0.1 | [29] |
| FeCoCrNiMo0.2 | Gas atomization | HVOF | 15–45 | FCC | FCC Oxides | 356 HV0.2 | [26] |
| AlCoCrFeNiTi | Gas atomization | APS | 42.9 | BCC1 BCC2 | BCC1 BCC2 Oxide | 599HV0.1 | [42] |
| Al1.3FeCoCrNiMo0.5 | Gas atomization | APS | 25 | BCC | FCC BCC Oxide | 650 HV0.05 | [30] |
| Al1.3FeCoCrNiMo0.5 | Gas atomization | HVOF | 25 | BCC | FCC BCC Oxide | 750 HV0.05 | [31] |
| AlCoCrFeMo | n/a | HVOF | 27 | BCC1(AlCoCrFe) BCC2(CrMo) | BCC1 BCC2 Oxides | 702 HV0.3 | [39] |
| CoCrNiTiW | Ball milling at 200 rpm for 10 h | HVOF | 22.7– 27.6 | BCC1 BCC2 | BCC1 BCC2 Oxides | 863–1025 HV0.3 | [40] |
| AlCoCrFeNiTi | High-energy ball milling at 400–700 rpm for 14 h | APS | - | BCC1 FCC(Ni) HCP(Ti) | BCC1 BCC2 FCC Oxide | 476 HV0.1 | [29] |
| Oxygen Flow Rate, L/min | Propane Flow Rate, L/min | Spray Distance, mm | Powder Feed Rate, rpm | Number of Cycles | Torch Velocity, m/min |
|---|---|---|---|---|---|
| 250 | 60 | 150 | 5.2 | 6 | 5 |
| Preparation Method | Chemical Composition, Mass.% | Average Particles Size, μm | Phase Composition | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Ni | Cr | Mo | Fe | Co | Al | Si | |||
| Blending (at 300 rpm) | 18.9 | 16.7 | 16.2 | 18.1 | 18.9 | 11.1 | - | 45 | BCC (Mo); BCC (Fe); BCC (Cr); FCC (Ni); FCC (Al); HCP (Co) |
| Milling (at 600 rpm) | 18.9 | 16.7 | 16.2 | 18.1 | 19.0 | 11.1 | - | 20 | BCC; BCC (Mo) |
| Gas atomization (0–63 μm) | 19.0 | 16.4 | 16.5 | 18.0 | 18.9 | 11.1 | 0.1 | 25 | BCC |
| Gas atomization (45–63 μm) | 19.0 | 16.4 | 16.7 | 17.9 | 18.9 | 11.1 | 0.1 | 50 | |
| Casting (nominal alloy’s composition) | 19.0 | 16.5 | 16.7 | 17.9 | 18.8 | 11.1 | 0.1 | - | B2; σ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Semikolenov, A.; Mamaev, N.; Larionova, T.; Shalnova, S.; Tolochko, O. Effect of Powder Preparation of FeNiCoCrMo0.5Al1.3 High-Entropy Alloy on the Phase Composition and Properties of High-Velocity Oxy-Fuel-Sprayed Coatings. J. Manuf. Mater. Process. 2024, 8, 280. https://doi.org/10.3390/jmmp8060280
Semikolenov A, Mamaev N, Larionova T, Shalnova S, Tolochko O. Effect of Powder Preparation of FeNiCoCrMo0.5Al1.3 High-Entropy Alloy on the Phase Composition and Properties of High-Velocity Oxy-Fuel-Sprayed Coatings. Journal of Manufacturing and Materials Processing. 2024; 8(6):280. https://doi.org/10.3390/jmmp8060280
Chicago/Turabian StyleSemikolenov, Anton, Nikolay Mamaev, Tatiana Larionova, Svetlana Shalnova, and Oleg Tolochko. 2024. "Effect of Powder Preparation of FeNiCoCrMo0.5Al1.3 High-Entropy Alloy on the Phase Composition and Properties of High-Velocity Oxy-Fuel-Sprayed Coatings" Journal of Manufacturing and Materials Processing 8, no. 6: 280. https://doi.org/10.3390/jmmp8060280
APA StyleSemikolenov, A., Mamaev, N., Larionova, T., Shalnova, S., & Tolochko, O. (2024). Effect of Powder Preparation of FeNiCoCrMo0.5Al1.3 High-Entropy Alloy on the Phase Composition and Properties of High-Velocity Oxy-Fuel-Sprayed Coatings. Journal of Manufacturing and Materials Processing, 8(6), 280. https://doi.org/10.3390/jmmp8060280







