Enhancing the Mechanical Properties of Transient-Liquid-Phase Bonded Inconel 617 to Stainless Steel 310 through Altering Process Parameters and Homogenisation
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
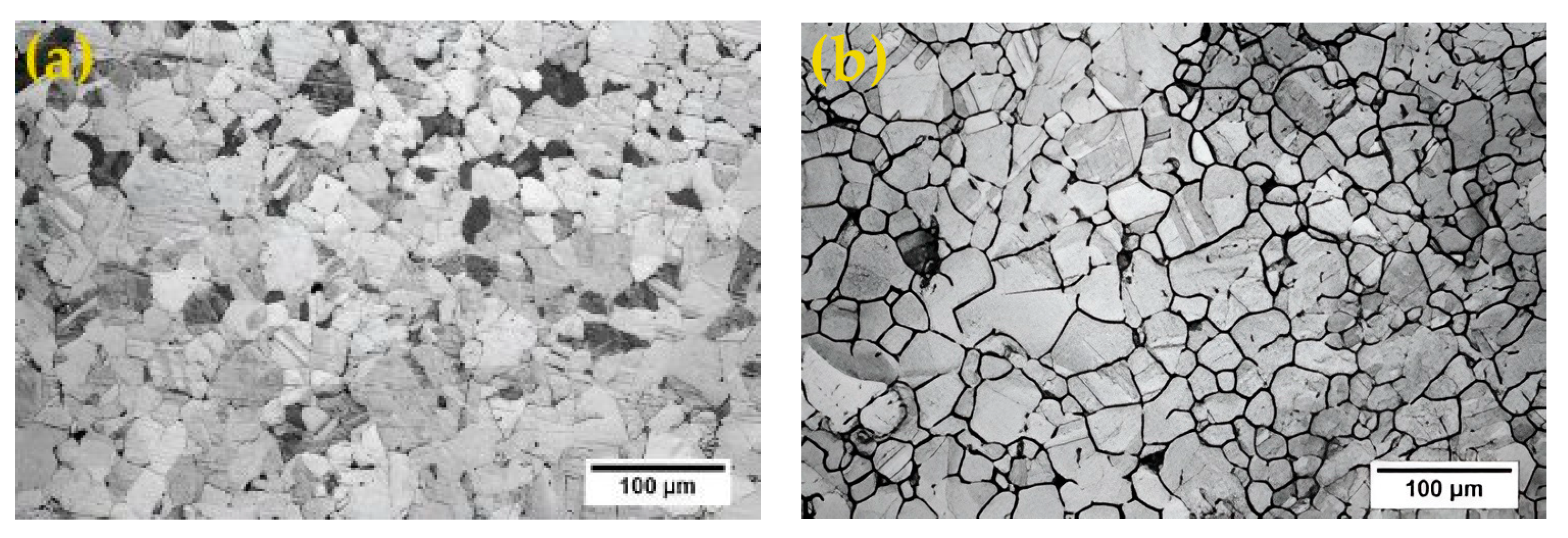
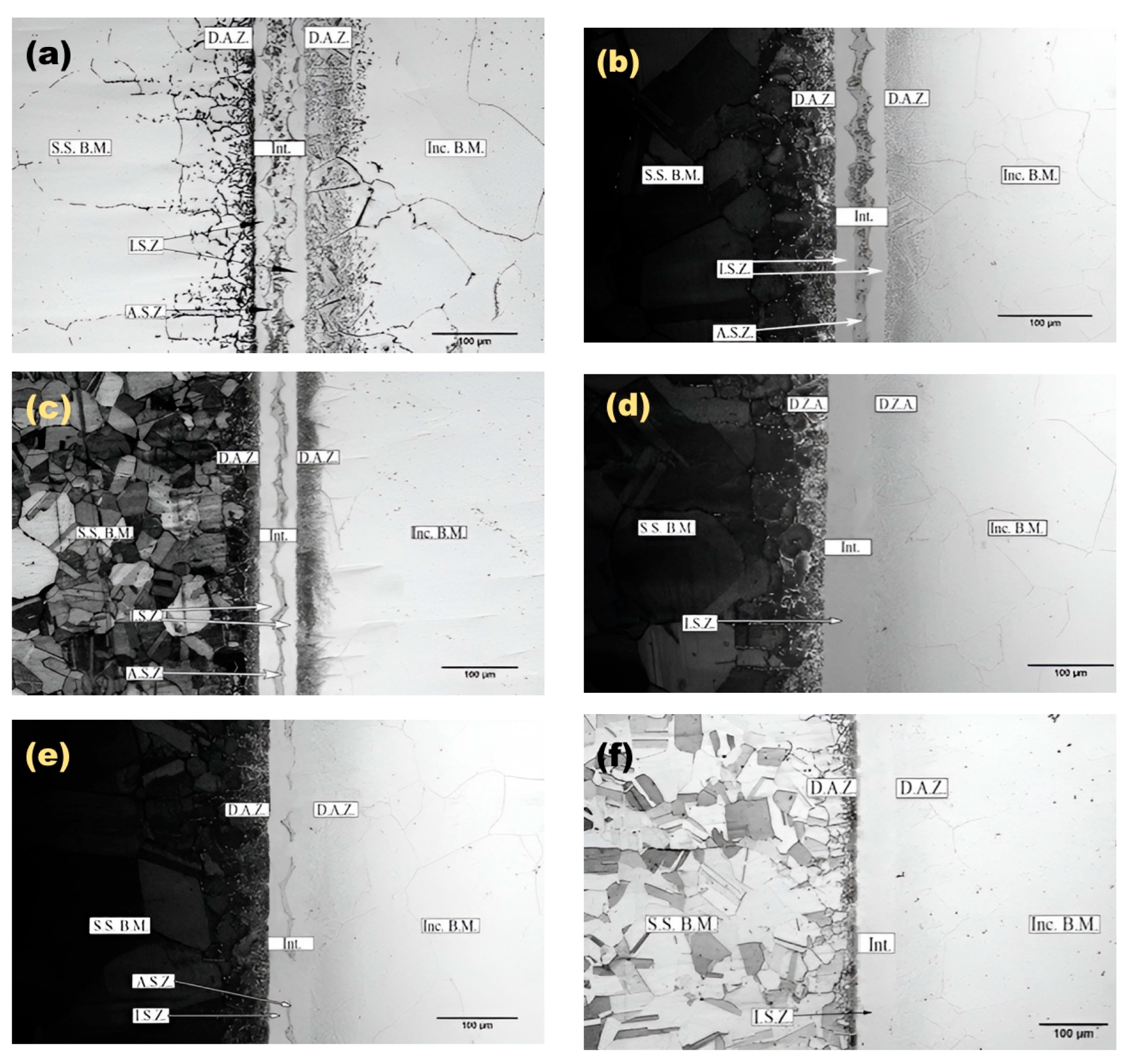
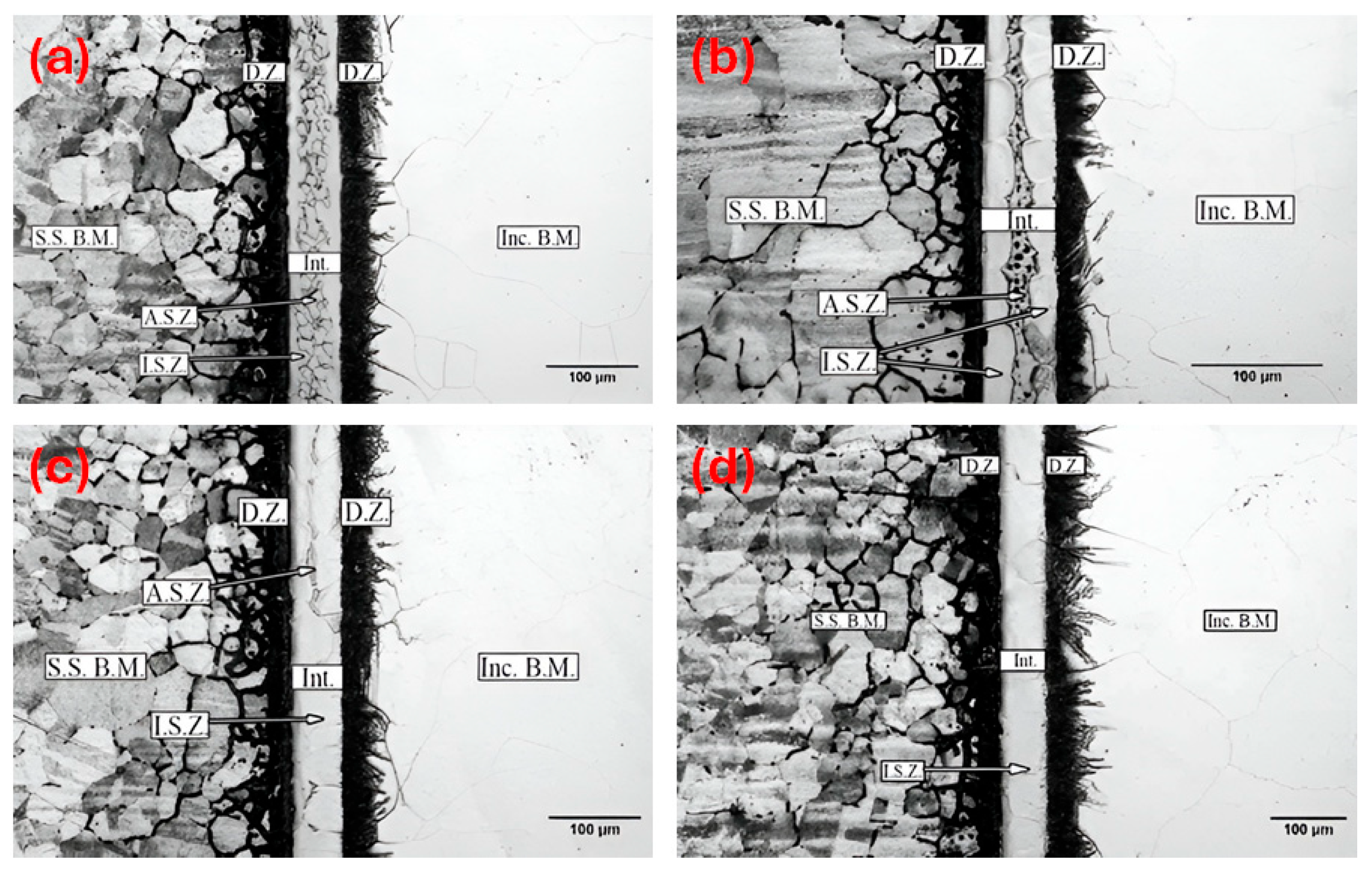
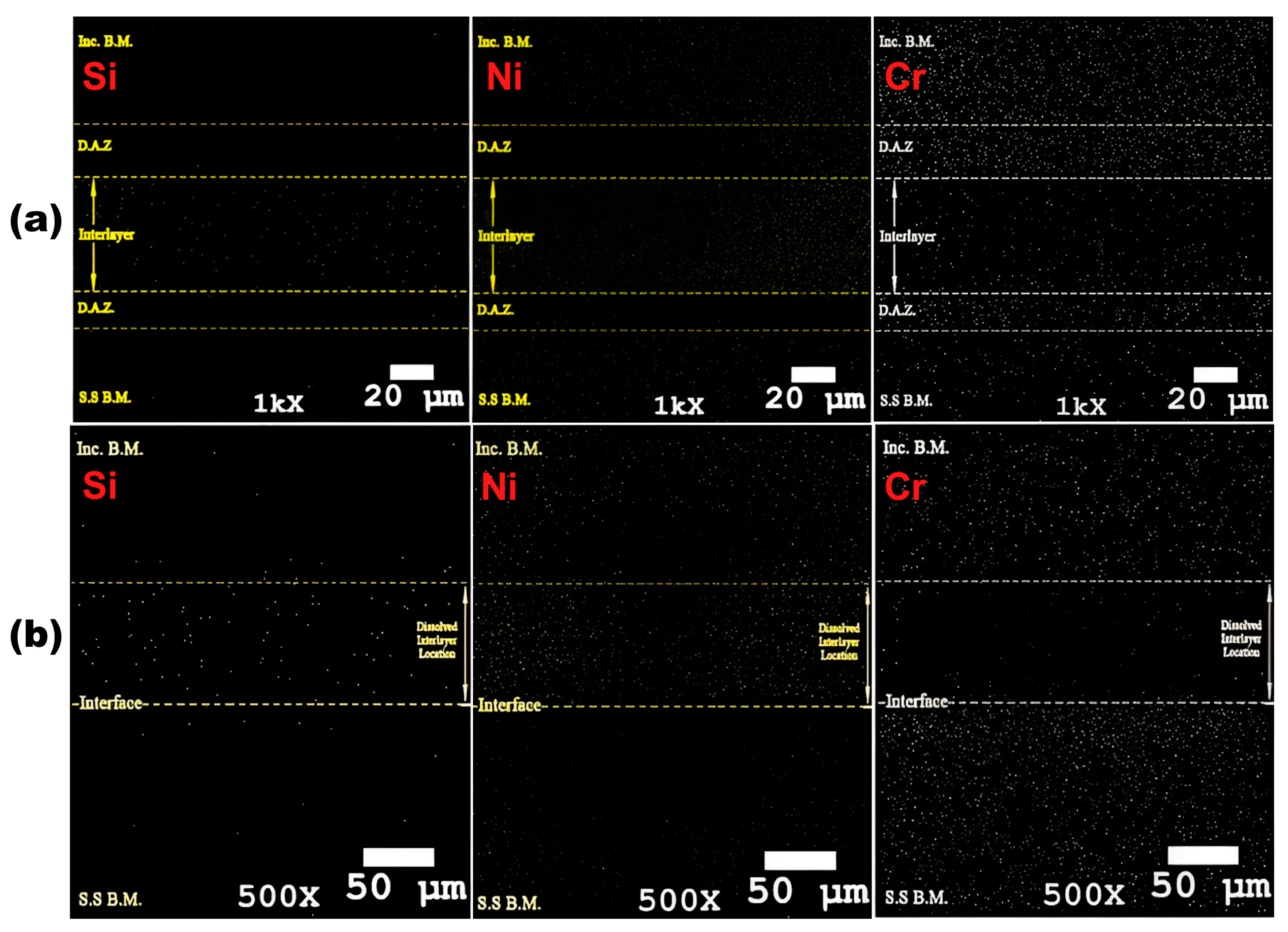
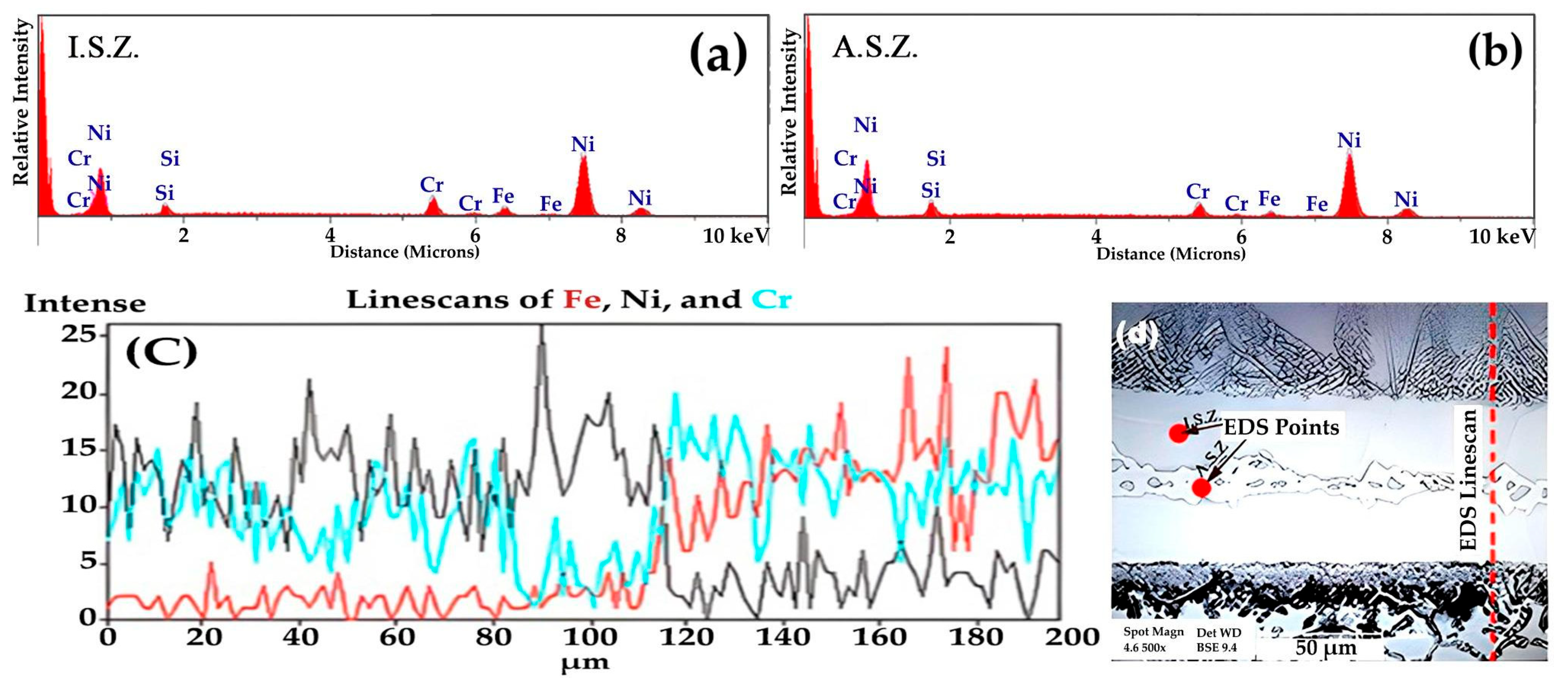
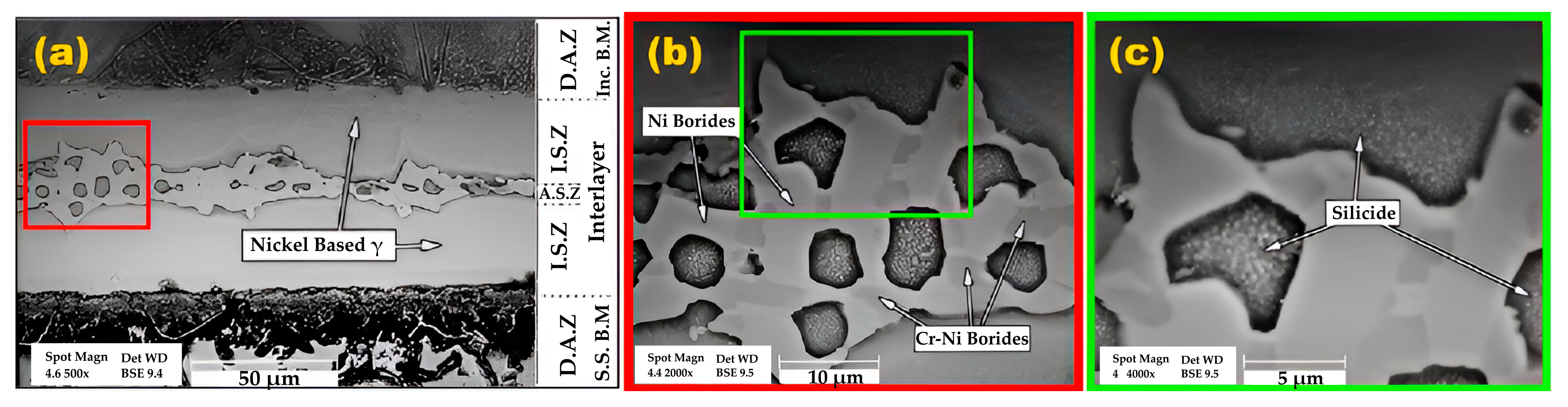
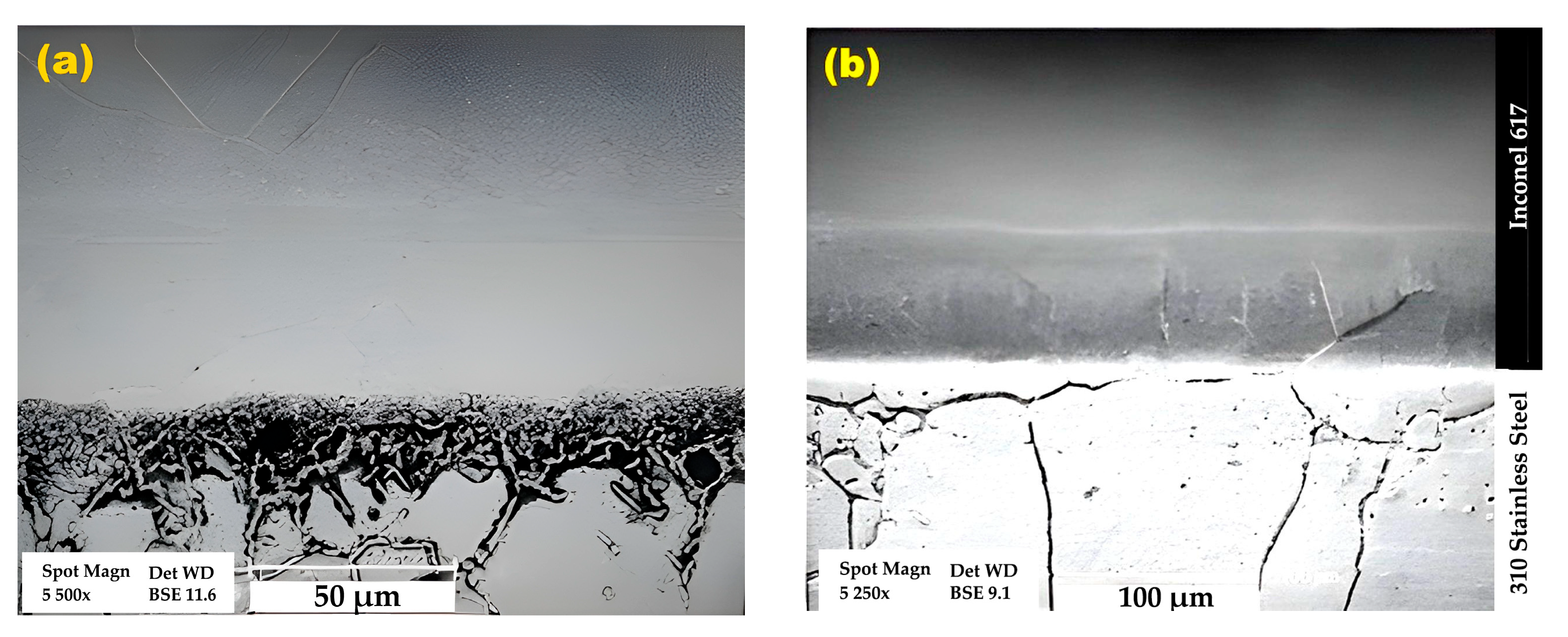
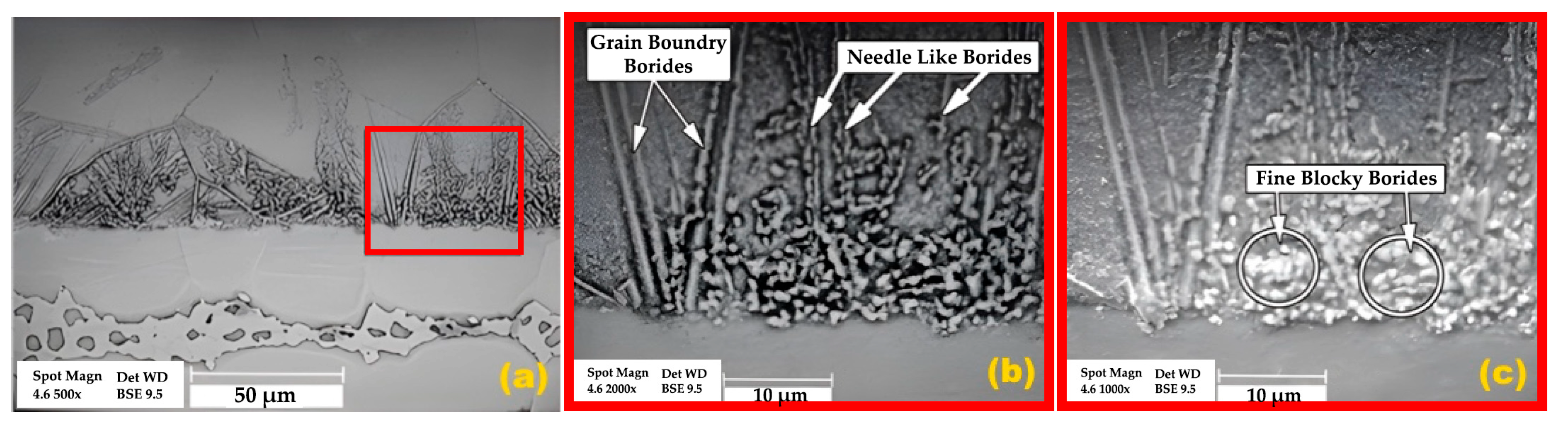
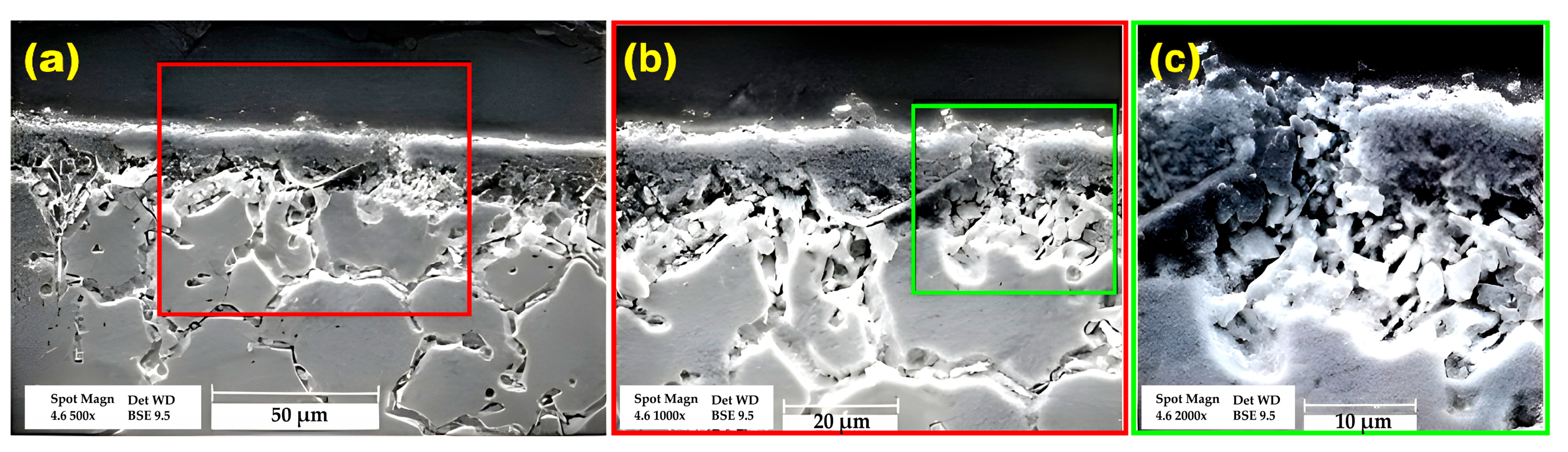
3.1. Microstructural Evolution
3.2. Mechanical Characterisation
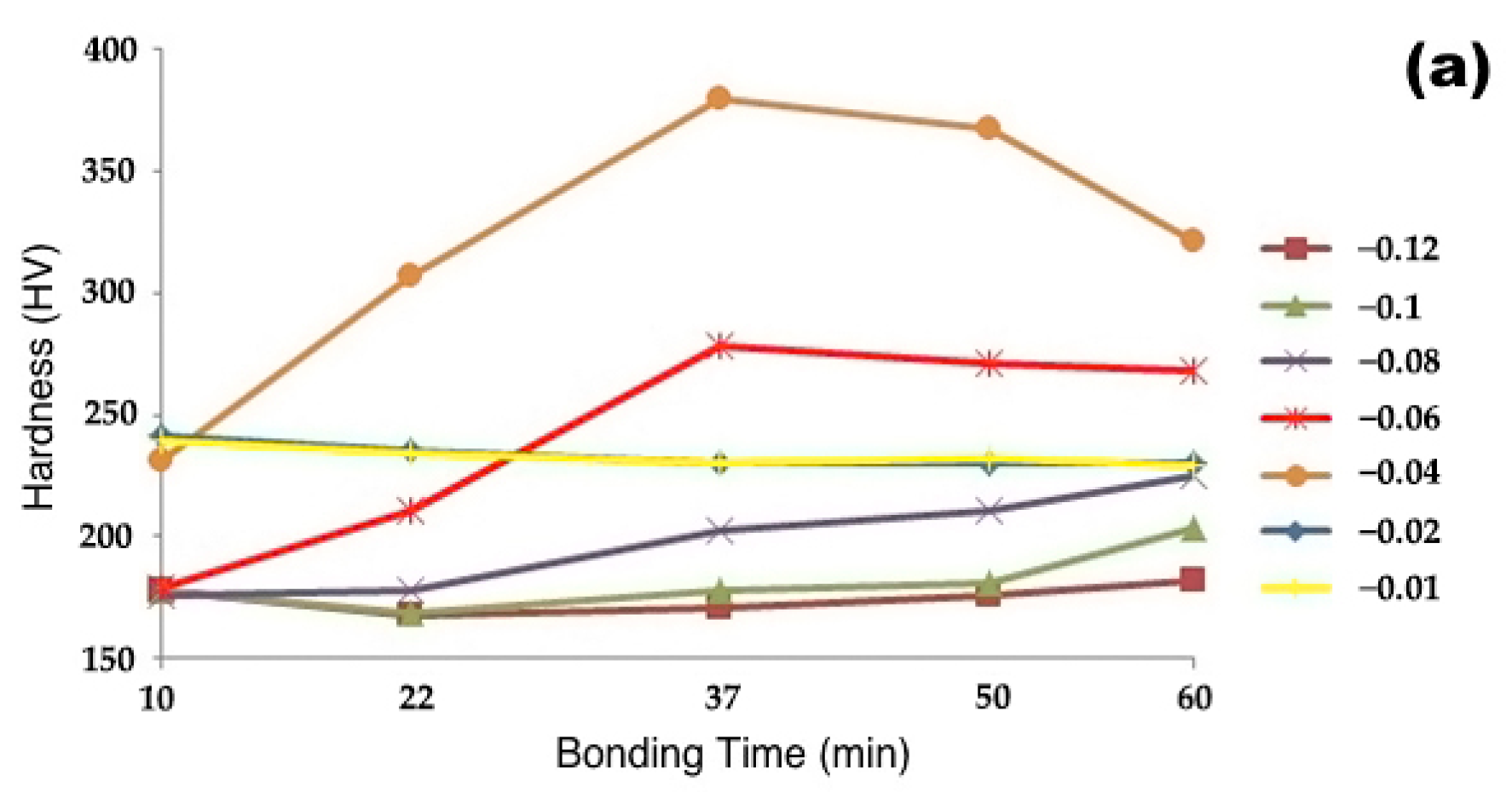
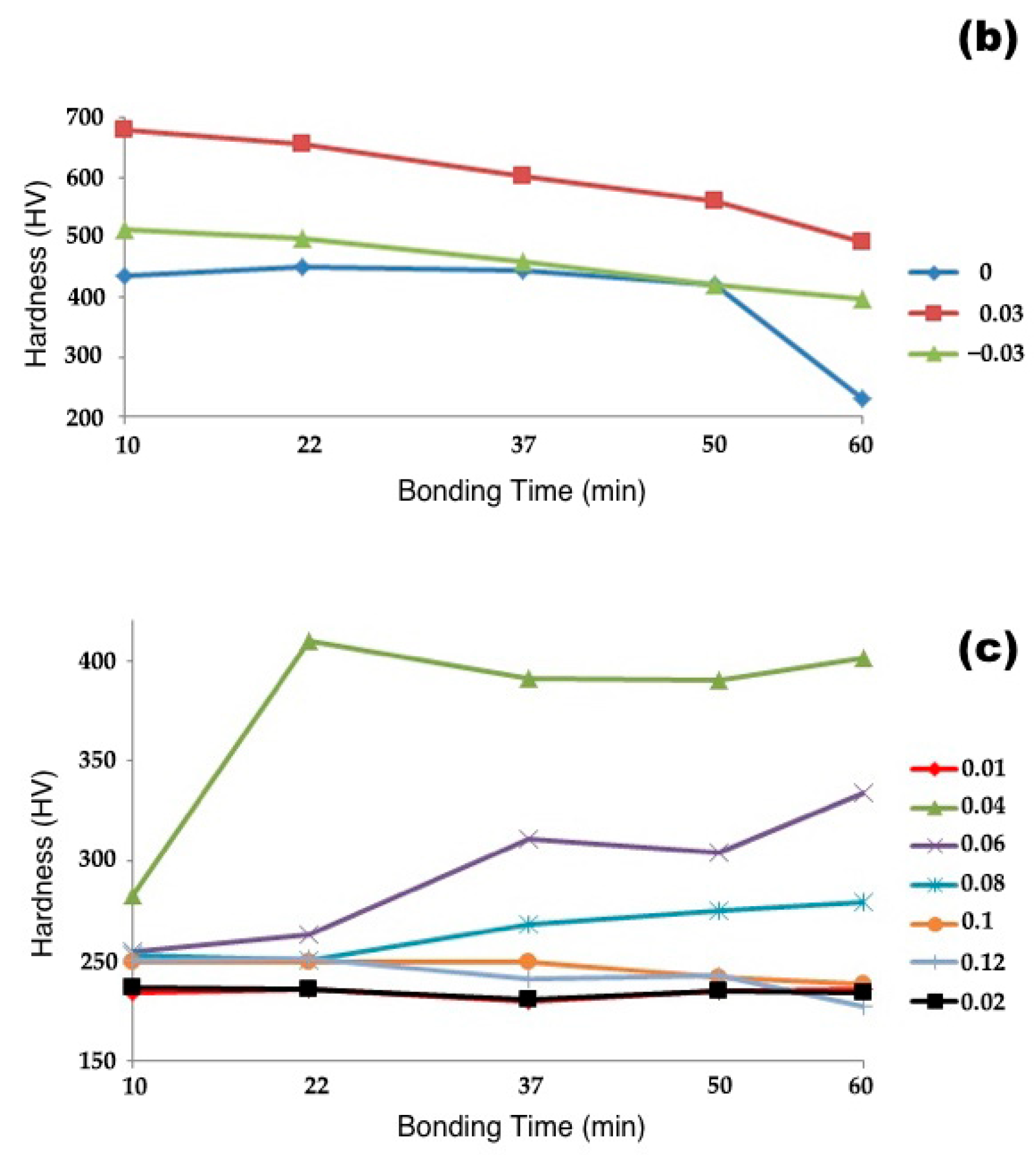
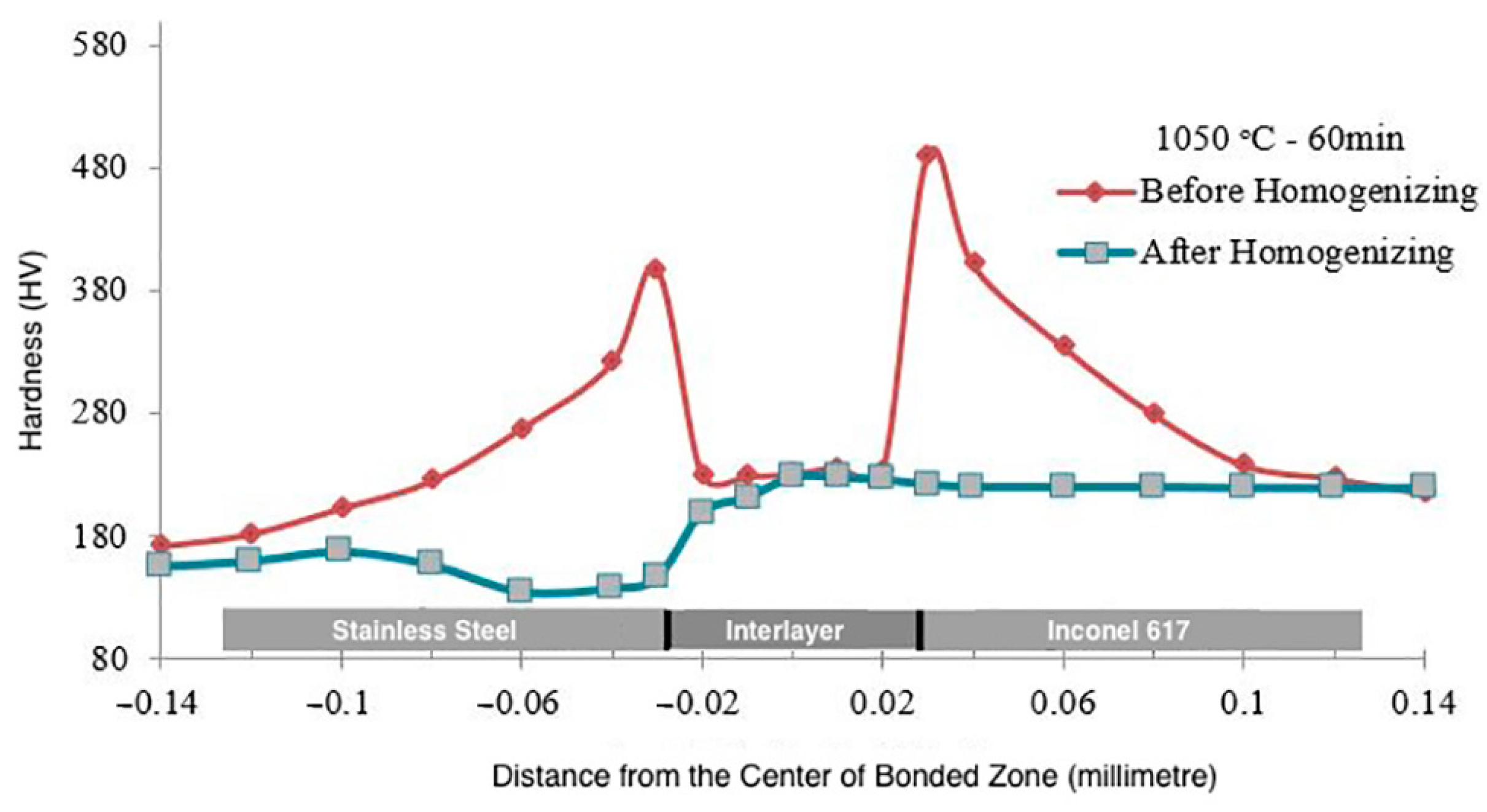
3.2.1. Microhardness
3.2.2. Shear Strength
4. Conclusions
- Complete isothermal solidification occurred in samples bonded at 1050 °C for 60 min and 1100 °C for 50 min.
- Increasing bonding time and temperature expanded the isothermal solidification zone, reducing eutectic phase content. Higher temperatures and longer times increased diffusion-affected zone, lowering eutectic phase concentration.
- The athermal solidified zone contained nickel-rich boride, nickel–chromium-rich boride, silicide phases, and nickel-based solid solution. The isothermal solidified zone is comprised of nickel-based proeutectoid solid solution.
- Homogenisation treatment eliminated concentration gradients in the diffusion-affected zone, enhancing the mechanical properties of bonded joints.
- As the bonding time and temperature rose, the rate of hardness variation decreased, and the shear strength of the bonded specimens increased.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ganjeh, E.; Kaflou, A.; Shirvani, K. High temperature shear and thermal aging behavior of dissimilar transient liquid phase bonded Hastelloy X to Ni3Al intermetallic compound. Intermetallics 2023, 159, 107916. [Google Scholar] [CrossRef]
- Qin, H.; Kuang, T.; Li, Q.; Yue, X.; Gao, H.; Liu, F.; Yi, Y. Stress concentration induced by the crystal orientation in the transient-liquid-phase bonded joint of single-crystalline Ni3Al. Materials 2019, 12, 2765. [Google Scholar] [CrossRef] [PubMed]
- Kokabi, D.; Kaflou, A.; Gholamipour, R.; Pouranvari, M. Microstructural Evaluation during dissimilar transient liquid phase bonding of TiAl/Ni-based superalloy. J. Alloys Compd. 2020, 853, 153999. [Google Scholar] [CrossRef]
- Alhazaa, A.; Haneklaus, N. Diffusion bonding and transient liquid phase (TLP) bonding of type 304 and 316 austenitic stainless steel—A review of similar and dissimilar material joints. Metals 2020, 10, 613. [Google Scholar] [CrossRef]
- Zhang, C.; Shirzadi, A. Fail-safe joints between copper alloy (C18150) and nickel-based superalloy (GH4169) made by transient liquid phase (TLP) bonding and using boron-nickel (BNi-2) interlayer. Metals 2021, 11, 1504. [Google Scholar] [CrossRef]
- Sah, I.; Hwang, J.B.; Kim, E.S. Creep behavior of diffusion-welded alloy 617. Metals 2021, 11, 830. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, X.; Zhang, L.; Zhang, S.; Feng, J. Experimental and numerical study of transient liquid phase diffusion bonded dz40m superalloys. Crystals 2021, 11, 479. [Google Scholar] [CrossRef]
- Zhang, L.X.; Chang, Q.; Sun, Z.; Xue, Q.; Feng, J.C. Effects of Boron and silicon on microstructural evolution and mechanical properties of transient liquid phase bonded GH3039/IC10 joints. J. Manuf. Process. 2019, 38, 167–173. [Google Scholar] [CrossRef]
- Farzadi, A.; Esmaeili, H.; Mirsalehi, S.E. Transient liquid phase bonding of Inconel 617 superalloy: Effect of filler metal type and bonding time. Weld. World 2019, 48, 1–11. [Google Scholar] [CrossRef]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H. Transient liquid phase bonding of wrought IN718 nickel based superalloy using standard heat treatment cycles: Microstructure and mechanical properties. Mater. Des. 2013, 50, 694–701. [Google Scholar] [CrossRef]
- Lin, Y.; Jiangtao, X.; Yajie, D.; Jin, R.; Junmiao, S.; Jinglong, L. Microstructure and mechanical properties in the TLP joint of FeCoNiTiAl and Inconel 718 alloys using BNi2 filler. J. Mater. Sci. Technol. 2021, 61, 176–185. [Google Scholar] [CrossRef]
- AlHazaa, A.; Alhoweml, I.; Shar, M.A.; Hezam, M.; Abdo, H.S.; AlBrithen, H. Transient liquid phase bonding of Ti-6Al-4V and Mg-AZ31 alloys using Zn coatings. Materials 2019, 12, 769. [Google Scholar] [CrossRef] [PubMed]
- Gale, W.F.; Butts, D.A. Transient liquid phase bonding. Sci. Technol. Weld. Join. 2004, 9, 283–300. [Google Scholar] [CrossRef]
- Duvall, D.S.; Owczarski, W.A.; Paulonis, D.F. Tlp Bonding: A New Method for Joining Heat Resistant Alloys. Weld. J. 1974, 53, 203–214. [Google Scholar]
- Malekan, A.; Farvizi, M.; Mirsalehi, S.E.; Saito, N.; Nakashima, K. Holding time influence on creep behavior of transient liquid phase bonded joints of Hastelloy X. Mater. Sci. Eng. 2020, 772, 138694. [Google Scholar] [CrossRef]
- Doroudi, A.; Pilehrood, A.E.; Mohebinia, M.; Dastgheib, A.; Rajabi, A.; Omidvar, H. Effect of the isothermal solidification completion on the mechanical properties of Inconel 625 transient liquid phase bond by changing bonding temperature. J. Mater. Res. Technol. 2020, 9, 10355–10365. [Google Scholar] [CrossRef]
- Mobarakeh, V.S.; Niroumand, B.; Atapour, M.; Shamanian, M. Effects of Transient Liquid Phase Bonding Time on Microstructure, Mechanical and Corrosion Properties During Bonding of Inconel 617/AISI 310 Stainless Steel. Metallogr. Microstruct. Anal. 2023, 12, 714–729. [Google Scholar] [CrossRef]
- Mobarakeh, V.S.; Atapour, M.; Niroumand, B.; Shamanian, M. Effect of Bonding Temperature on Microstructure and Mechanical Properties of Dissimilar Joint Between Inconel 617 and Stainless Steel 310. Metallogr. Microstruct. Anal. 2021, 10, 419–429. [Google Scholar] [CrossRef]
- Ghaderi, S.; Karimzadeh, F.; Ashrafi, A.; Hosseini, S.H. Effect of pressure, temperature and homogenisation on the dissolution behavior and mechanical properties of IN718/AISI 304 during transient liquid phase bonding. J. Manuf. Process. 2020, 60, 213–226. [Google Scholar] [CrossRef]
- Di Luozzo, N.; Fontana, M.; Arcondo, B. Transient liquid phase bonding of steel using an Fe-B interlayer: Microstructural analysis. J. Mater. Sci. 2008, 43, 4938–4944. [Google Scholar] [CrossRef]
- American Welding Society. Brazing Handbook, 5th ed.; AWS: Miami, FL, USA, 2007; pp. 1–19. [Google Scholar]
- E92-23; Standard Test Methods for Vickers Hardness and Knoop Hardness of Metallic Materials. ASTM: West Conshohocken, PA, USA, 2023.
- Shah Hosseini, H.; Shamanian, M.; Kermanpur, A. Characterisation of microstructures and mechanical properties of Inconel 617/310 stainless steel dissimilar welds. Mater. Charact. 2011, 62, 425–431. [Google Scholar] [CrossRef]
- Lin, T.S.; Li, H.X.; He, P.; Yang, X.; Huang, Y.; Li, L.; Han, L. Effect of bonding parameters on microstructures and properties during TLP bonding of Ni-based super alloy. Trans. Nonferrous Met. Soc. China 2012, 22, 2112–2117. [Google Scholar] [CrossRef]
- Yuan, X.; Kim, M.B.; Kang, C.Y. Characterization of transient-liquid-phase-bonded joints in a duplex stainless steel with a Ni-Cr-B insert alloy. Mater. Charact. 2009, 60, 1289–1297. [Google Scholar] [CrossRef]
- Porter, D.A.; Easterling, K.E.; Sherif, M.Y. Phase Transformations in Metals and Alloys, 4th ed.; CRC Press: New York, NY, USA, 2021; pp. 63–111. [Google Scholar] [CrossRef]
- Arafin, M.A.; Medraj, M.; Turner, D.P.; Bocher, P. Effect of alloying elements on the isothermal solidification during TLP bonding of SS 410 and SS 321 using a BNi-2 interlayer. Mater. Chem. Phys. 2007, 106, 109–119. [Google Scholar] [CrossRef]
- Cook, G.O.; Sorensen, C.D. Overview of transient liquid phase and partial transient liquid phase bonding. J. Mater. Sci. 2011, 46, 5305–5323. [Google Scholar] [CrossRef]
- Pouranvari, M.; Ekrami, A.; Kokabi, A.H.; Han, H.N. Microstructural characteristics of a cast IN718 superalloy bonded by isothermal solidification. Met. Mater. Int. 2013, 19, 1091–1099. [Google Scholar] [CrossRef]
- Jalilvand, V.; Omidvar, H.; Shakeri, H.R.; Rahimipour, M.R. Microstructural evolution during transient liquid phase bonding of Inconel 738LC using AMS 4777 filler alloy. Mater. Charact. 1970, 75, 20–28. [Google Scholar] [CrossRef]
- Jalilian, F.; Jahazi, M.; Drew, R.A.L. Microstructural evolution during transient liquid phase bonding of Inconel 617 using Ni-Si-B filler metal. Mater. Sci. Eng. 2006, A 423, 269–281. [Google Scholar] [CrossRef]
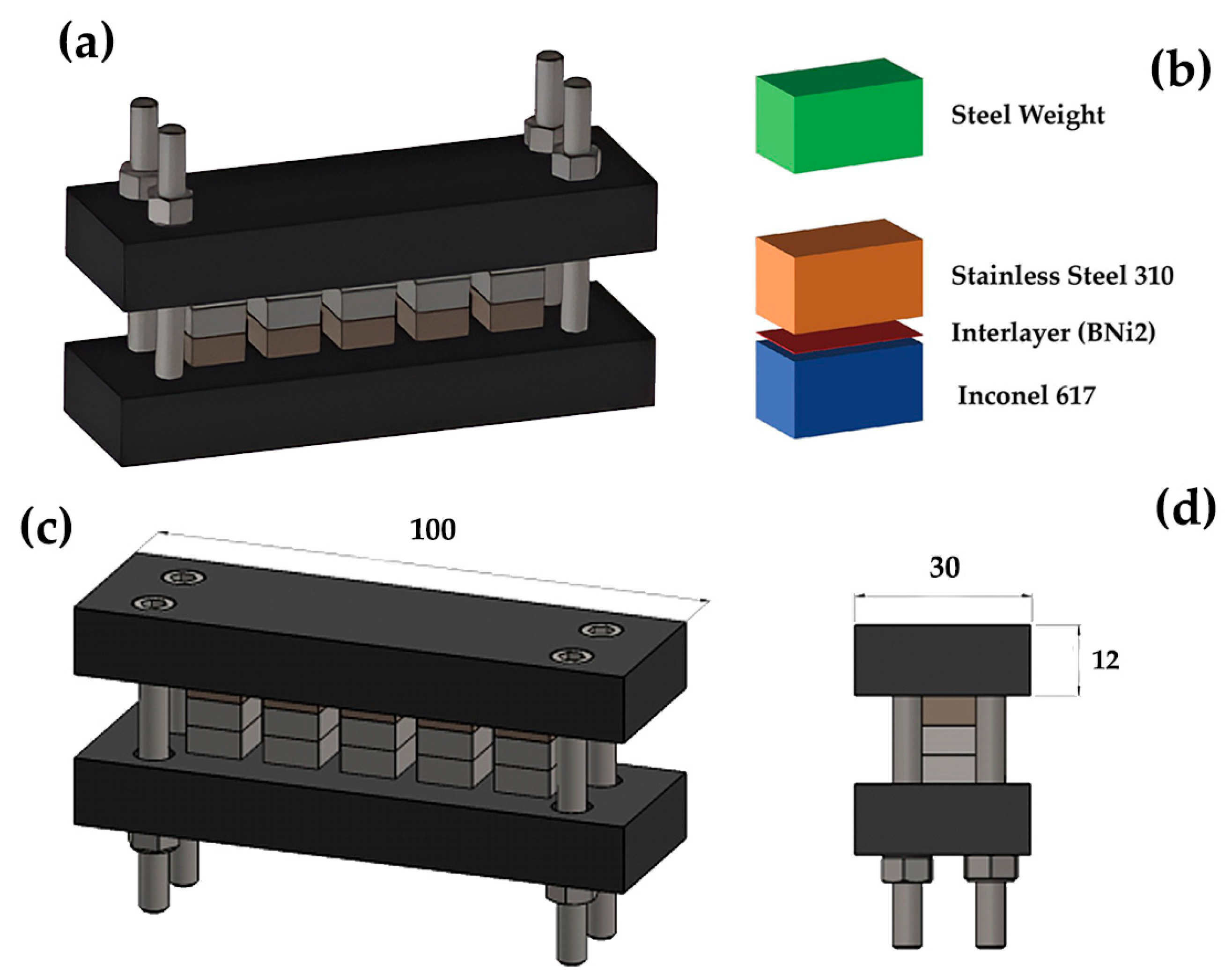
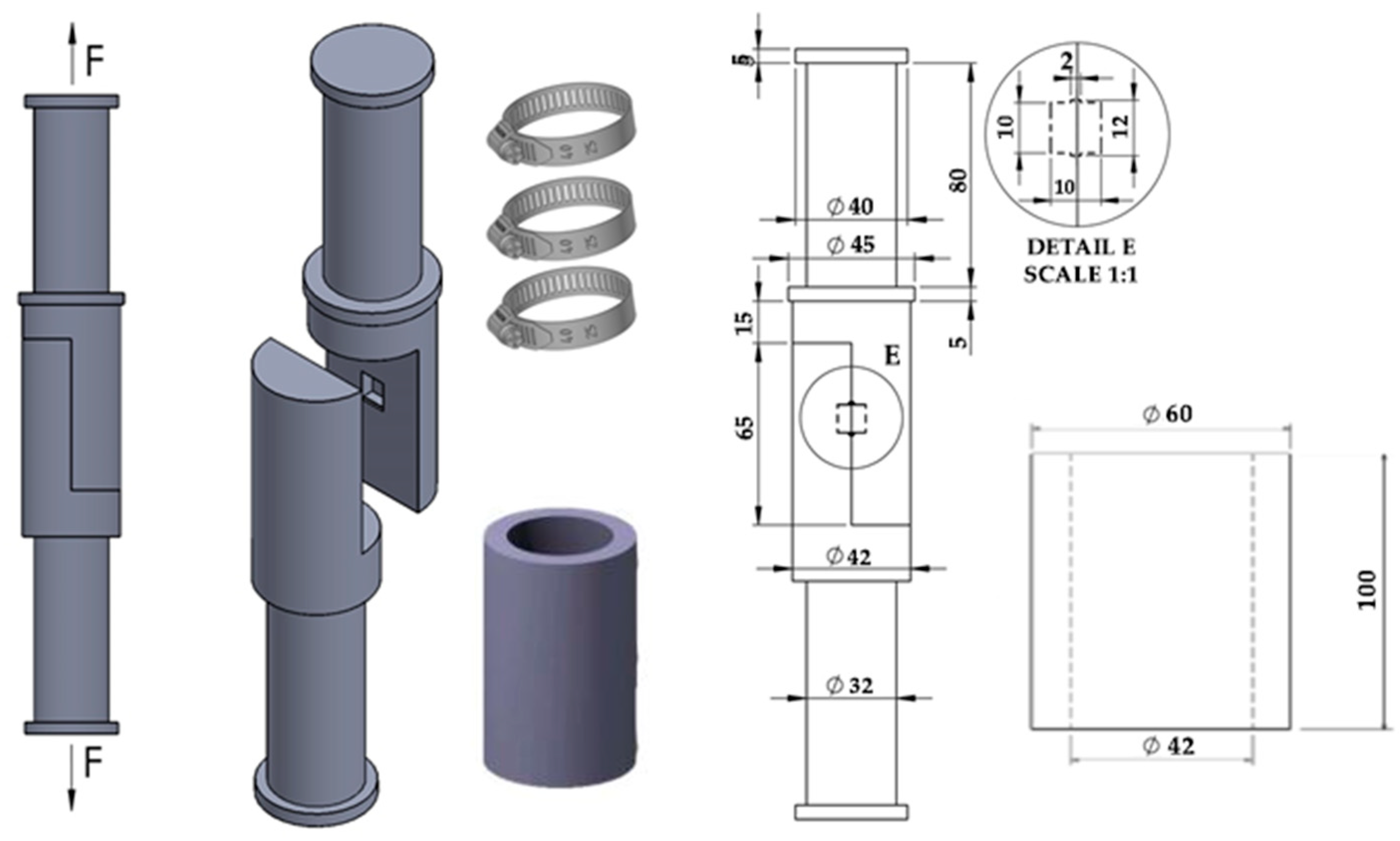



| Materials | C | Cr | Ni | Mo | Mn | Si | Fe | Ti | Co | Al | Nb | B |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inc 617 | 0.06 | 22.26 | Rem | 8.73 | 0.04 | 0.02 | 0.4 | 0.32 | 12.36 | 0.87 | 0.06 | - |
| SS 310 | 0.09 | 25.74 | 20.31 | 0.09 | 1.4 | 0.96 | Rem | - | - | - | - | - |
| BNi2 | 0.07 | 7.23 | Rem | - | - | 4.6 | - | - | - | - | - | 2.97 |
| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Temperature (°C) | 1050 | 1050 | 1050 | 1050 | 1050 | 1100 | 1100 | 1100 | 1100 |
| Time (min) | 10 | 22 | 37 | 50 | 60 | 10 | 22 | 37 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dehghan, A.; Emadi, R.; Asghari, Y.; Emadi, H.; Lotfian, S. Enhancing the Mechanical Properties of Transient-Liquid-Phase Bonded Inconel 617 to Stainless Steel 310 through Altering Process Parameters and Homogenisation. J. Manuf. Mater. Process. 2024, 8, 143. https://doi.org/10.3390/jmmp8040143
Dehghan A, Emadi R, Asghari Y, Emadi H, Lotfian S. Enhancing the Mechanical Properties of Transient-Liquid-Phase Bonded Inconel 617 to Stainless Steel 310 through Altering Process Parameters and Homogenisation. Journal of Manufacturing and Materials Processing. 2024; 8(4):143. https://doi.org/10.3390/jmmp8040143
Chicago/Turabian StyleDehghan, Arash, Rahmatollah Emadi, Yunes Asghari, Hosein Emadi, and Saeid Lotfian. 2024. "Enhancing the Mechanical Properties of Transient-Liquid-Phase Bonded Inconel 617 to Stainless Steel 310 through Altering Process Parameters and Homogenisation" Journal of Manufacturing and Materials Processing 8, no. 4: 143. https://doi.org/10.3390/jmmp8040143
APA StyleDehghan, A., Emadi, R., Asghari, Y., Emadi, H., & Lotfian, S. (2024). Enhancing the Mechanical Properties of Transient-Liquid-Phase Bonded Inconel 617 to Stainless Steel 310 through Altering Process Parameters and Homogenisation. Journal of Manufacturing and Materials Processing, 8(4), 143. https://doi.org/10.3390/jmmp8040143







