Current Trends in Dissimilar Diffusion Bonding of Titanium Alloys to Stainless Steels, Aluminium and Magnesium
Abstract
1. Introduction
1.1. Dissimilar Joining of Titanium Alloys
1.2. Diffusion Bonding Process
2. Effect of Bonding Parameters
2.1. Bonding Time and Temperature
2.2. Pressure and Surface Roughness
2.3. Interlayer Composition and Thickness
3. Mechanical Performance of Joints
4. Mechanisms of Bond Formation
5. Key Challenges and Future Direction
Author Contributions
Funding
Conflicts of Interest
References
- Peters, M.; Hemptenmacher, J.; Kumpfert, J.; Leyens, C. Structure and Properties of Titanium and Titanium Alloys. In Titanium and Titanium Alloys; Wiley: Weinheim, Germany, 2005. [Google Scholar]
- van Beek, J.A.; Kodentsov, A.A.; van Loo, F.J.J. Phase equilibria in the CuFeTi system at 1123 K. J. Alloys Compd. 1995, 217, 97–103. [Google Scholar] [CrossRef]
- De Viteri, V.S.; Fuentes, E. Titanium and Titanium Alloys as Biomaterials. In Tribology - Fundamentals and Advancements; Intech: London, UK, 2013. [Google Scholar]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys; Wiley: Weinheim, Germany, 2003. [Google Scholar]
- Balasubramanian, M. Characterization of diffusion-bonded titanium alloy and 304 stainless steel with Ag as an interlayer. Int. J. Adv. Manuf. Technol. 2016, 82, 153–162. [Google Scholar] [CrossRef]
- Davies, P.; Johal, A.; Davies, H.; Marchisio, S. Powder interlayer bonding of titanium alloys: Ti-6Al-2Sn-4Zr-6Mo and Ti-6Al-4V. Int. J. Adv. Manuf. Technol. 2019, 103, 441–452. [Google Scholar] [CrossRef]
- Bache, M.R.; Tuppen, S.J.; Voice, W.E.; Lee, H.G.; Aspinwall, D.K. Novel low cost procedure for fabrication of diffusion bonds in Ti 6/4. Mater. Sci. Technol. 2009, 25, 39–49. [Google Scholar] [CrossRef]
- Kah, P.; Suoranta, R.; Martikainen, J.; Magnus, C. Techniques for joining dissimilar materials: Metals and polymers. Rev. Adv. Mater. Sci. 2014, 16, 229–237. [Google Scholar]
- Wulandari, W.; Brooks, G.A.; Rhamdhani, M.A.; Monaghan, B.J. Magnesium: Current and Alternative Production Routes. In Proceedings of the Australian Conference on Chemical Engineering, Hilton Adelaide, Australia, 26–29 September 2010. [Google Scholar]
- Akhtar, T.S.; Cooke, K.O.; Khan, T.I.; Shar, M.A. Nanoparticle enhanced eutectic reaction during diffusion brazing of aluminium to magnesium. Nanomaterials 2019, 9, 370. [Google Scholar] [CrossRef] [PubMed]
- Lighweight Materials 2016 Annual Report. Available online: https://www.energy.gov/sites/prod/files/2017/11/f46/FY_2016_APR_Consolidated_Report_compliant-102617.pdf. (accessed on 19 April 2020).
- Sameer Kumar, D.; Tara Sasanka, C. Magnesium and its alloys. In Lightweight and Sustainable Materials for Automotive Applications; Taylor and Francis: Oxfordshire, UK, 2017; ISBN 9781498756884. [Google Scholar]
- Wang, M.; Wang, J.; Ke, W. Microelectronics Reliability Corrosion behavior of Sn-3.0Ag-0.5Cu lead-free solder joints. Microelectron. Reliab. 2017, 73, 69–75. [Google Scholar] [CrossRef]
- Ireland, R.; Arronche, L.; Saponara, V. La Electrochemical investigation of galvanic corrosion between aluminum 7075 and glass fiber / epoxy composites modified with carbon nanotubes. Compos. B. Eng. 2012, 43, 183–194. [Google Scholar] [CrossRef]
- Atieh, A.M.; Khan, T.I. TLP bonding of Ti-6Al-4V and Mg-AZ31 alloys using pure Ni electro-deposited coats. J. Mater. Process. Technol. 2014, 214, 3158–3168. [Google Scholar] [CrossRef]
- Atieh, A.M.; Khan, T.I. Effect of process parameters on semi-solid TLP bonding of Ti-6Al-4V to Mg-AZ31. J. Mater. Sci. 2013, 8, 6737–6745. [Google Scholar] [CrossRef]
- Atieh, A.M.; Khan, T.I. Effect of Interlayer Thickness on Joint Formation Between Ti-6Al-4V and Mg-AZ31 Alloys. J. Mater. Eng. Perform. 2014, 23, 4042–4054. [Google Scholar] [CrossRef]
- Shanmugarajan, B.; Padmanabham, G. Fusion welding studies using laser on Ti-SS dissimilar combination. Opt. Lasers Eng. 2012, 50, 1621–1627. [Google Scholar] [CrossRef]
- Cooke, K.O. Electrodeposited Nanocomposite Coatings: Principles and Applications; Nova Science Publisher: New York, NY, USA, 2014; Volume 3, ISBN 9781629485690. [Google Scholar]
- Tanaka, K.; Nakazawa, T.; Sakairi, K.; Sato, Y.; Kokawa, H.; Omori, T.; Ishida, K. Feasibility of Iridium Containing Nickel Based Superalloy Tool to Friction Stir Spot Welding of High Strength Steel. In Minerals, Metals and Materials Series; Springer: Berlin, Germany, 2017. [Google Scholar]
- Prangnell, P.B.; Bakavos, D. Novel Approaches to Friction Spot Welding Thin Aluminium Automotive Sheet. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2010. [Google Scholar]
- Simões, S.; Viana, F.; Ramos, A.S.; Teresa Vieira, M.; Vieira, M.F. Microstructural characterization of dissimilar titanium alloys joints using Ni/Al nanolayers. Metals. 2018, 8, 715. [Google Scholar] [CrossRef]
- Cooke, K.O.; Alhazaa, A.; Atieh, A.M. Dissimilar Welding and Joining of Magnesium Alloys: Principles and Application. In Magnesium—The Wonder Element for Engineering/Biomedical Applications; Intech: London, UK, 2019. [Google Scholar]
- Jalali, A.; Atapour, M.; Shamanian, M.; Vahman, M. Transient liquid phase (TLP) bonding of Ti-6Al-4V/UNS 32750 super duplex stainless steel. J. Manuf. Process. 2018, 33, 194–202. [Google Scholar] [CrossRef]
- Mo, D.F.; Song, T.F.; Fang, Y.J.; Jiang, X.S.; Luo, C.Q.; Simpson, M.D.; Luo, Z.P. A review on diffusion bonding between titanium alloys and stainless steels. Adv. Mater. Sci. Eng. 2018, 2018, 8701890. [Google Scholar] [CrossRef]
- AlHazaa, A.; Alhoweml, I.; Shar, M.A.; Hezam, M.; Abdo, H.S.; AlBrithen, H. Transient liquid phase bonding of Ti-6Al-4V and Mg-AZ31 alloys using Zn coatings. Materials 2019, 12, 769. [Google Scholar] [CrossRef]
- Caiazzo, F.; Curcio, F.; Daurelio, G.; Minutolo, F.M.C. Ti6Al4V sheets lap and butt joints carried out by CO2 laser: Mechanical and morphological characterization. J. Mater. Process. Technol. 2004, 149, 546–552. [Google Scholar] [CrossRef]
- Balasubramanian, T.S.; Balasubramanian, V.; Muthumanikkam, M.A. Fatigue performance of gas tungsten arc, electron beam, and laser beam welded Ti-6Al-4V alloy joints. J. Mater. Eng. Perform. 2011, 20, 1620–1630. [Google Scholar] [CrossRef]
- Atieh, A.M.; Allaf, R.M.; AlHazaa, A.; Barghash, M.; Mubaydin, H. Effect of Pre- and Post-Weld Shot Peening on the Mechanical and Tribological Properties of TIG Welded Al 6061-T6 Alloy. Trans. Can. Soc. Mech. Eng. 2017, 41, 197–209. [Google Scholar] [CrossRef]
- Habisch, S.; Böhme, M.; Peter, S.; Grund, T.; Mayr, P. The effect of interlayer materials on the joint properties of diffusion-bonded aluminium and magnesium. Metals. 2018, 8, 138. [Google Scholar] [CrossRef]
- Alemán, B.; Gutiérrez, L.; Urcola, J.J. Interface microstructures in diffusion bonding of titanium alloys to stainless and low alloy steels. Mater. Sci. Technol. 1993, 9, 633–641. [Google Scholar] [CrossRef]
- Li, S.; Chen, Y.; Kang, J.; Huang, Y.; Gianetto, J.A.; Yin, L. Interfacial microstructures and mechanical properties of dissimilar titanium alloy and steel friction stir butt-welds. J. Manuf. Process. 2019, 40, 160–168. [Google Scholar] [CrossRef]
- Choi, J.W.; Liu, H.; Ushioda, K.; Fujii, H. Dissimilar friction stir welding of immiscible titanium and magnesium. Materialia 2019, 7, 100389. [Google Scholar] [CrossRef]
- Malikov, A.; Vitoshkin, I.; Orishich, A.; Filippov, A.; Karpov, E. Effect of the aluminum alloy composition (Al-Cu-Li or Al-Mg-Li) on structure and mechanical properties of dissimilar laser welds with the Ti-Al-V alloy. Opt. Laser Technol. 2020, 126, 106135. [Google Scholar] [CrossRef]
- Malikov, A.; Vitoshkin, I.; Orishich, A.; Filippov, A.; Karpov, E. Microstructure and mechanical properties of laser welded joints of Al-Cu-Li and Ti-Al-V alloys. J. Manuf. Process. 2020, 53, 201–212. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.K.; Zhou, J.P.; Xue, R.L.; Sun, D.Q.; Li, H.M. Characterization of laser beam offset welding of titanium to steel with 38Zn-61Cu alloy filler. Opt. Laser Technol. 2020, 127, 106195. [Google Scholar] [CrossRef]
- Shen, J.; Li, B.; Hu, S.; Zhang, H.; Bu, X. Comparison of single-beam and dual-beam laser welding of Ti–22Al–25Nb/TA15 dissimilar titanium alloys. Opt. Laser Technol. 2017, 93, 118–126. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.P.; Sun, D.Q.; Gu, X.Y. Nd: YAG laser welding of dissimilar metals of titanium alloy to stainless steel without filler metal based on a hybrid connection mechanism. J. Mater. Res. Technol. 2019, 9, 1662–1672. [Google Scholar] [CrossRef]
- Xu, W.F.; Jun, M.A.; Luo, Y.X.; Fang, Y.X. Microstructure and high-temperature mechanical properties of laser beam welded TC4/TA15 dissimilar titanium alloy joints. Trans. Nonferrous Met. Soc. China. 2020, 30, 160–170. [Google Scholar] [CrossRef]
- Ma, Z.; Jin, Y.; Ji, S.; Meng, X.; Ma, L.; Li, Q. A general strategy for the reliable joining of Al/Ti dissimilar alloys via ultrasonic assisted friction stir welding. J. Mater. Sci. Technol. 2019, 35, 94–99. [Google Scholar] [CrossRef]
- Xiong, X.; Zhao, P.; Ren, R.; Zhang, Z.; Cui, X.; Ji, S. Enhanced resistance-welding hybrid joints of titanium alloy/thermoplastic composites using a carbon-nanotube lamina. Diam. Relat. Mater. 2020, 101, 107611. [Google Scholar] [CrossRef]
- Esfahani Yeganeh, V.; Li, P. Effect of beam offset on microstructure and mechanical properties of dissimilar electron beam welded high temperature titanium alloys. Mater. Des. 2017, 124, 78–86. [Google Scholar] [CrossRef]
- Atieh, A.M.; Khan, T.I. Application of Ni and Cu nanoparticles in transient liquid phase (TLP) bonding of Ti-6Al-4V and Mg-AZ31 alloys. J. Mater. Sci. 2014, 49, 7648–7658. [Google Scholar] [CrossRef]
- Atieh, A.M.; Khan, T.I. Transient liquid phase (TLP) brazing of Mg – AZ31 and Ti – 6Al – 4V using Ni and Cu sandwich foils. Sci. Technol. Weld. Join. 2014, 19, 333–342. [Google Scholar] [CrossRef]
- AlHazaa, A.; Khan, T.I. Diffusion bonding of Al7075 to Ti–6Al–4V using Cu coatings and Sn–3.6Ag–1Cu interlayers. J. Alloys Compd. 2010, 494, 351–358. [Google Scholar] [CrossRef]
- Cooke, K.O. A study of the effect of nanosized particles on transient liquid phase diffusion bonding al6061 metal-matrix composite (MMC) using Ni/Al 2O 3 Nanocomposite Interlayer. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2012, 43, 627–634. [Google Scholar] [CrossRef]
- Gale, W.F.; Butts, D.A. Transient liquid phase bonding. Sci. Technol. Weld. Join. 2004, 9, 283–300. [Google Scholar] [CrossRef]
- Zhuang, W.D.; Eagar, T.W. Transient liquid-phase bonding using coated metal powders. Weld. J. (Miami, Fla) 1997, 76, 157s. [Google Scholar]
- Tuah-Poku, I.; Dollar, M.; Massalski, T. A study of the transient liquid phase bonding process applied to a Ag/Cu/Ag sandwich joint. Metall. Trans. A Trans. A 1988, 19, 675–686. [Google Scholar] [CrossRef]
- Kuntz, M.L.; Zhou, Y.; Corbin, S.F. A study of transient liquid-phase bonding of Ag-Cu using differential scanning calorimetry. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2006, 37, 2493. [Google Scholar] [CrossRef]
- Velmurugan, C.; Senthilkumar, V.; Sarala, S.; Arivarasan, J. Low temperature diffusion bonding of Ti-6Al-4V and duplex stainless steel. J. Mater. Process. Technol. 2016, 234, 272–279. [Google Scholar] [CrossRef]
- Kurt, B.; Orhan, N.; Evin, E.; Çalik, A. Diffusion bonding between Ti-6Al-4V alloy and ferritic stainless steel. Mater. Lett. 2007, 61, 1747–1750. [Google Scholar] [CrossRef]
- Balasubramanian, M. Development of processing windows for diffusion bonding of Ti-6Al-4V titanium alloy and 304 stainless steel with silver as intermediate layer. Trans. Nonferrous Met. Soc. China. 2015, 25, 2932–2938. [Google Scholar] [CrossRef]
- Li, H.; Zhang, C.; Liu, H.B.; Li, M.Q. Bonding interface characteristic and shear strength of diffusion bonded Ti-17 titanium alloy. Trans. Nonferrous Met. Soc. China. 2015, 25, 80–87. [Google Scholar] [CrossRef]
- Kenevisi, M.S.; Mousavi Khoie, S.M. An investigation on microstructure and mechanical properties of Al7075 to Ti-6Al-4V Transient Liquid Phase (TLP) bonded joint. Mater. Des. 2012, 38, 19–25. [Google Scholar] [CrossRef]
- Kenevisi, M.S.; Mousavi Khoie, S.M.; Alaei, M. Microstructural evaluation and mechanical properties of the diffusion bonded Al/Ti alloys joint. Mech. Mater. 2013, 64, 69–75. [Google Scholar] [CrossRef]
- Rajakumar, S.; Balasubramanian, V. Diffusion bonding of titanium and AA 7075 aluminum alloy dissimilar joints—process modeling and optimization using desirability approach. Int. J. Adv. Manuf. Technol. 2016, 86, 1095–1112. [Google Scholar] [CrossRef]
- Nakagawa, N.; Hwang, H.Y.; Muller, D.A. Why some interfaces cannot be sharp. Nat. Mater. 2006, 5, 204–209. [Google Scholar] [CrossRef]
- Song, T.F.; Jiang, X.S.; Shao, Z.Y.; Fang, Y.J.; Mo, D.F.; Zhu, D.G.; Zhu, M.H. Microstructure and mechanical properties of vacuum diffusion bonded joints between Ti-6Al-4V titanium alloy and AISI316L stainless steel using Cu/Nb multi-interlayer. Vacuum 2017, 145, 68–76. [Google Scholar] [CrossRef]
- Li, S.X.; Tu, S.T.; Xuan, F.Z. A probabilistic model for prediction of bonding time in diffusion bonding. Mater. Sci. Eng. A 2005, 407, 250–255. [Google Scholar] [CrossRef]
- Zuruzi, A.S.; Li, H.; Dong, G. Effects of surface roughness on the diffusion bonding of Al alloy 6061 in air. Mater. Sci. Eng. A 1999, 270, 244–248. [Google Scholar] [CrossRef]
- Li, B.; Zhang, Z.; Shen, Y.; Hu, W.; Luo, L. Dissimilar friction stir welding of Ti-6Al-4V alloy and aluminum alloy employing a modified butt joint configuration: Influences of process variables on the weld interfaces and tensile properties. Mater. Des. 2014, 53, 838–848. [Google Scholar] [CrossRef]
- Wang, A.; Ohashi, O.; Ueno, K. Effect of surface asperity on diffusion bonding. Mater. Trans. 2006, 47, 179–184. [Google Scholar] [CrossRef]
- Samavatian, M.; Zakipour, S.; Paidar, M. Effect of bonding pressure on microstructure and mechanical properties of Ti-6Al-4V diffusion-bonded joint. Weld. World 2017, 61, 69–74. [Google Scholar] [CrossRef]
- Shirzadi, A.A.; Laik, A.; Tewari, R.; Orsborn, J.; Dey, G.K. Gallium-assisted diffusion bonding of stainless steel to titanium; microstructural evolution and bond strength. Materialia 2018, 4, 115–126. [Google Scholar] [CrossRef]
- Ghosh, M.; Bhanumurthy, K.; Kale, G.B.; Krishnan, J.; Chatterjee, S. Diffusion bonding of titanium to 304 stainless steel. J. Nucl. Mater. 2003, 322, 235–241. [Google Scholar] [CrossRef]
- Simões, S.; Ramos, A.S.; Viana, F.; Vieira, M.T.; Vieira, M.F. Joining of TiAl to steel by diffusion bonding with Ni/Ti reactive multilayers. Metals. 2016, 6, 96. [Google Scholar] [CrossRef]
- Cooke, K.O.; Richardson, A.; Khan, T.I.; Shar, M.A. High-Temperature Diffusion Bonding of Ti–6Al–4V and Super-Duplex Stainless Steel Using a Cu Interlayer Embedded with Alumina Nanoparticles. J. Manuf. Mater. Process. 2020, 4, 3. [Google Scholar] [CrossRef]
- He, P.; Yue, X.; Zhang, J.H. Hot pressing diffusion bonding of a titanium alloy to a stainless steel with an aluminum alloy interlayer. Mater. Sci. Eng. A 2008, 486, 171–176. [Google Scholar] [CrossRef]
- Deng, Y.; Sheng, G.; Yin, L. Impulse pressuring diffusion bonding of titanium to stainless steel using a copper interlayer. Xiyou Jinshu Cailiao Yu Gongcheng/Rare Met. Mater. Eng. 2015, 44, 1041–1045. [Google Scholar]
- Yuan, X.; Liang, X.; Li, X.; Hong, M.; Tang, K. Microstructure and mechanical property of brazed joints in titanium alloy and aluminum alloy combination with tin foil interlayer. Int. J. Precis. Eng. Manuf. 2015, 16, 1293–1297. [Google Scholar] [CrossRef]
- Kundu, S.; Ghosh, M.; Laik, A.; Bhanumurthy, K.; Kale, G.B.; Chatterjee, S. Diffusion bonding of commercially pure titanium to 304 stainless steel using copper interlayer. Mater. Sci. Eng. A 2005, 407, 154–160. [Google Scholar] [CrossRef]
- AlHazaa, A.; Khan, T.I.; Haq, I. Transient liquid phase (TLP) bonding of Al7075 to Ti-6Al-4V alloy. Mater. Charact. 2010, 61, 312–317. [Google Scholar] [CrossRef]
- Kundu, S.; Thirunavukarasu, G.; Chatterjee, S.; Mishra, B. Effect of Bonding Temperature on Phase Transformation of Diffusion-Bonded Joints of Duplex Stainless Steel and Ti-6Al-4V Using Nickel and Copper as Composite Intermediate Metals. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2015, 46, 5756–5771. [Google Scholar] [CrossRef]
- Zhang, Z.; Tan, C.; Zhao, X.; Chen, B.; Song, X.; Zhao, H. Influence of Cu coating thickness on interfacial reactions in laser welding-brazing of Mg to Ti. J. Mater. Process. Technol. 2018, 261, 61–73. [Google Scholar] [CrossRef]
- Auwal, S.T.; Ramesh, S.; Zhang, Z.; Yusof, F.; Liu, J.; Tan, C.; Manladan, S.M.; Tarlochan, F. Effect of copper-nickel interlayer thickness on laser welding-brazing of Mg/Ti alloy. Opt. Laser Technol. 2019, 115, 149–159. [Google Scholar] [CrossRef]
- Kundu, S.; Thirunavukarasu, G. Structure and properties correlation of diffusion bonded joint of duplex stainless steel and Ti–6Al–4V with and without Ni–17Cr–9Fe alloy interlayer. Weld. World 2016, 60, 793–811. [Google Scholar] [CrossRef]
- Ferrante, M.; Pigoretti, E.V. Diffusion bonding of Ti-6Al-4V to AISI 316L stainless steel: Mechanical resistance and interface microstructure. J. Mater. Sci. 2002, 37, 2825–2833. [Google Scholar] [CrossRef]
- Akca, E.; Gursel, A. The importance of interlayers in diffusion welding - A review. Period. Eng. Nat. Sci. 2015, 3, 12–16. [Google Scholar]
- Liu, J.; Tan, C.; Wu, L.; Zhao, X.; Zhang, Z.; Chen, B.; Song, X.; Feng, J. Butt laser welding-brazing of AZ31Mg alloy to Cu coated Ti-6Al-4V with AZ92 Mg based filler. Opt. Laser Technol. 2019, 117, 200–214. [Google Scholar] [CrossRef]
- Norouzi, E.; Atapour, M.; Shamanian, M.; Allafchian, A. Effect of bonding temperature on the microstructure and mechanical properties of Ti-6Al-4V to AISI 304 transient liquid phase bonded joint. Mater. Des. 2016, 99, 543–551. [Google Scholar] [CrossRef]
- Simões, S.; Viana, F.; Ventzke, V.; Koçak, M.; Sofia Ramos, A.; Teresa Vieira, M.; Vieira, M.F. Diffusion bonding of TiAl using Ni/Al multilayers. J. Mater. Sci. 2010, 45, 4351–4357. [Google Scholar] [CrossRef]
- Balasubramanian, M. Application of Box-Behnken design for fabrication of titanium alloy and 304 stainless steel joints with silver interlayer by diffusion bonding. Mater. Des. 2015, 77, 161–169. [Google Scholar] [CrossRef]
- Deng, Y.; Sheng, G.; Xu, C. Evaluation of the microstructure and mechanical properties of diffusion bonded joints of titanium to stainless steel with a pure silver interlayer. Mater. Des. 2013, 46, 84–87. [Google Scholar] [CrossRef]
- Afghahi, S.S.S.; Ekrami, A.; Farahany, S.; Jahangiri, A. Fatigue properties of temperature gradient transient liquid phase diffusion bonded Al7075-T6 alloy. Trans. Nonferrous Met. Soc. China (English Ed.) 2015, 25, 1073–1079. [Google Scholar] [CrossRef]
- Li, P.; Li, J.; Xiong, J.; Zhang, F.; Raza, S.H. Diffusion bonding titanium to stainless steel using Nb/Cu/Ni multi-interlayer. Mater. Charact. 2012, 68, 82–87. [Google Scholar] [CrossRef]
- Samavatian, M.; Khodabandeh, A.; Halvaee, A.; Amadeh, A.A. Transient liquid phase bonding of Al 2024 to Ti-6Al-4V alloy using Cu-Zn interlayer. Trans. Nonferrous Met. Soc. China. 2015, 25, 770–775. [Google Scholar] [CrossRef]
- Lee, M.K.; Lee, J.G.; Choi, Y.H.; Kim, D.W.; Rhee, C.K.; Lee, Y.B.; Hong, S.J. Interlayer engineering for dissimilar bonding of titanium to stainless steel. Mater. Lett. 2010, 64, 1105–1108. [Google Scholar] [CrossRef]
- Yuan, X.; Kim, M.B.; Kang, C.Y. Characterization of transient-liquid-phase-bonded joints in a duplex stainless steel with a Ni-Cr-B insert alloy. Mater. Charact. 2009, 60, 1289–1297. [Google Scholar] [CrossRef]
- Kenevisi, M.S.; Mousavi Khoie, S.M. A study on the effect of bonding time on the properties of Al7075 to Ti-6Al-4V diffusion bonded joint. Mater. Lett. 2012, 76, 144–146. [Google Scholar] [CrossRef]
- Anbarzadeh, A.; Sabet, H.; Abbasi, M. Effects of successive-stage Transient Liquid Phase (S-TLP) on microstructure and mechanical properties of Al2024 to Ti-6Al-4V joint. Mater. Lett. 2016, 178, 280–283. [Google Scholar] [CrossRef]
- Chang, S.Y.; Tsao, L.C.; Lei, Y.H.; Mao, S.M.; Huang, C.H. Brazing of 6061 aluminum alloy/Ti-6Al-4V using Al-Si-Cu-Ge filler metals. J. Mater. Process. Technol. 2012, 212, 8–14. [Google Scholar] [CrossRef]
- Kundu, S.; Sam, S.; Chatterjee, S. Evaluation of interface microstructure and mechanical properties of the diffusion bonded joints of Ti-6Al-4V alloy to micro-duplex stainless steel. Mater. Sci. Eng. A 2011, 528, 4910–4916. [Google Scholar] [CrossRef]
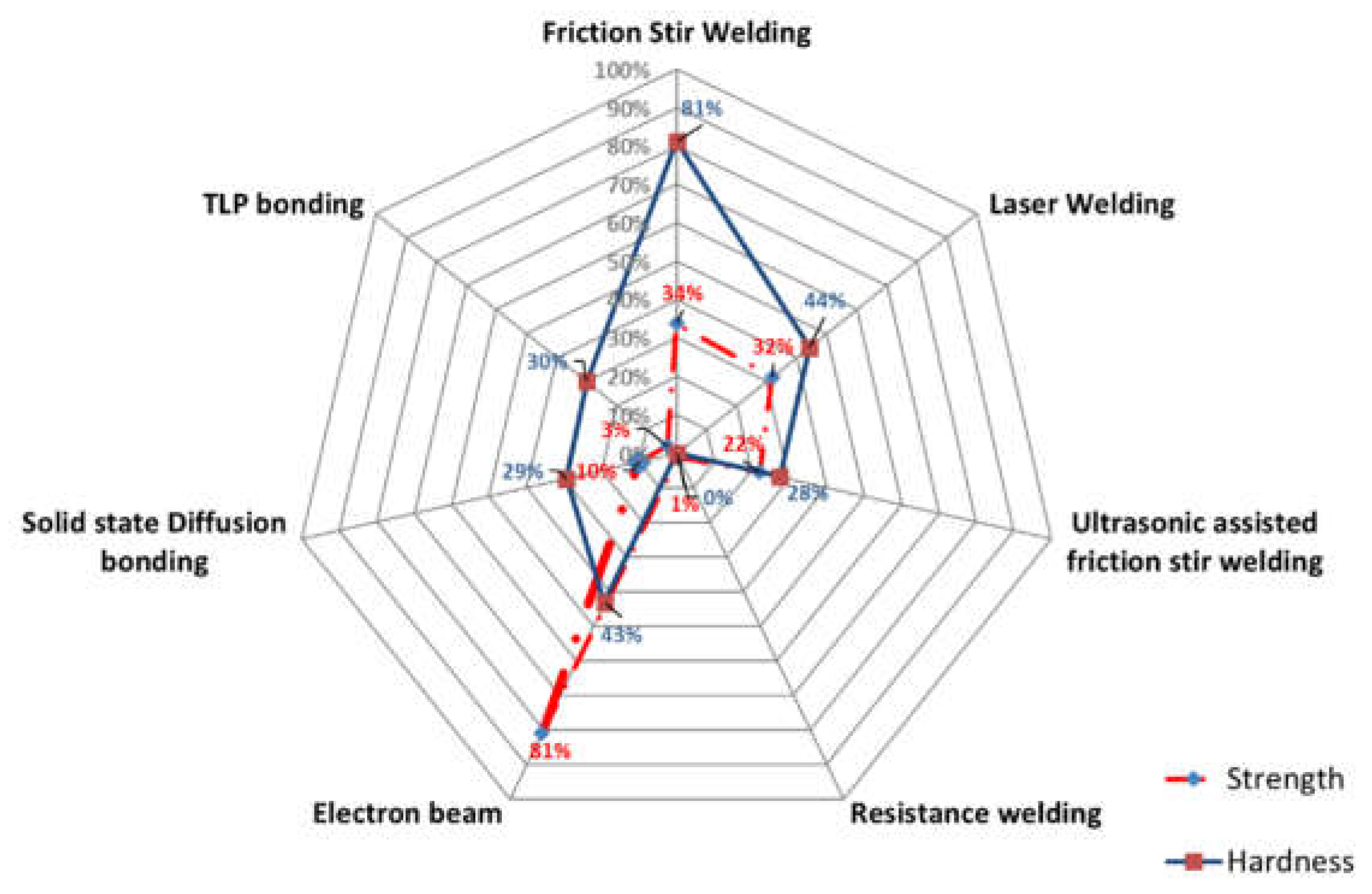
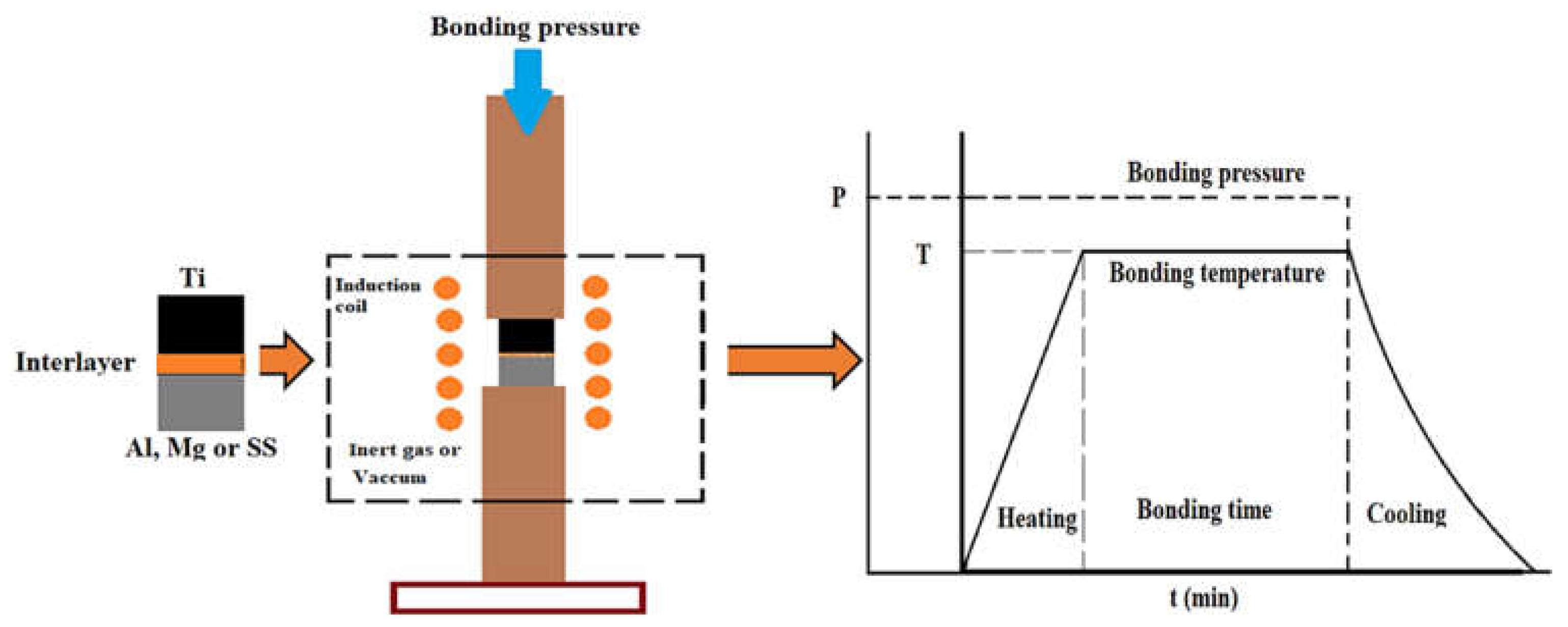
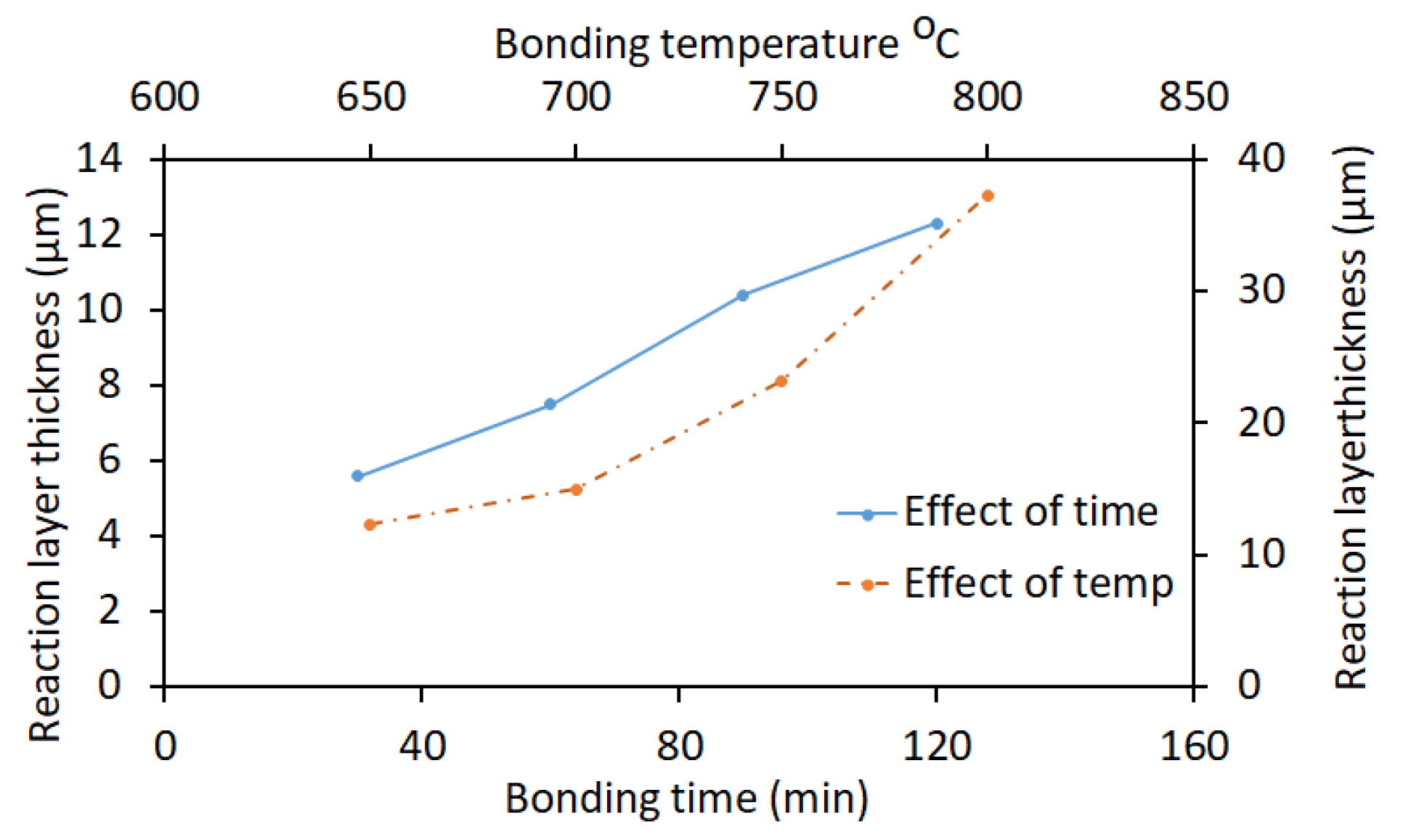
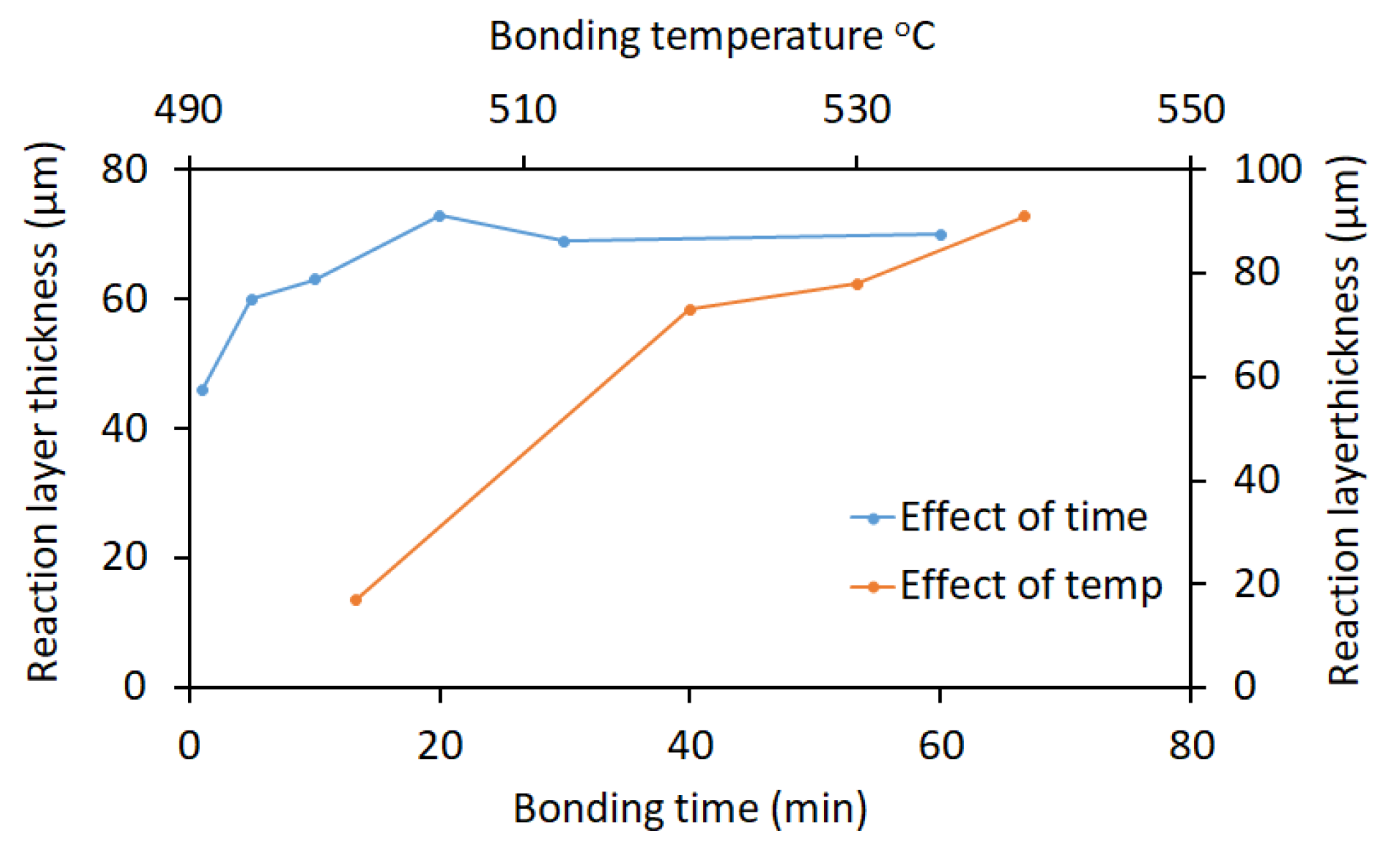
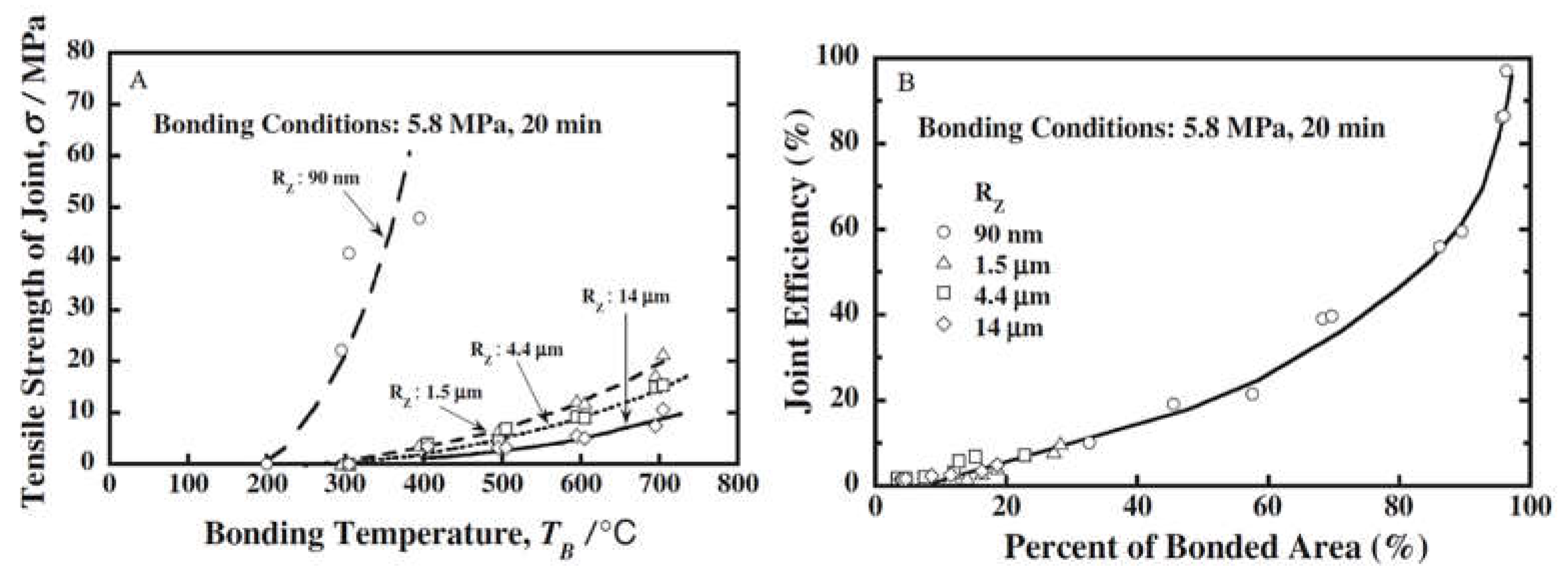
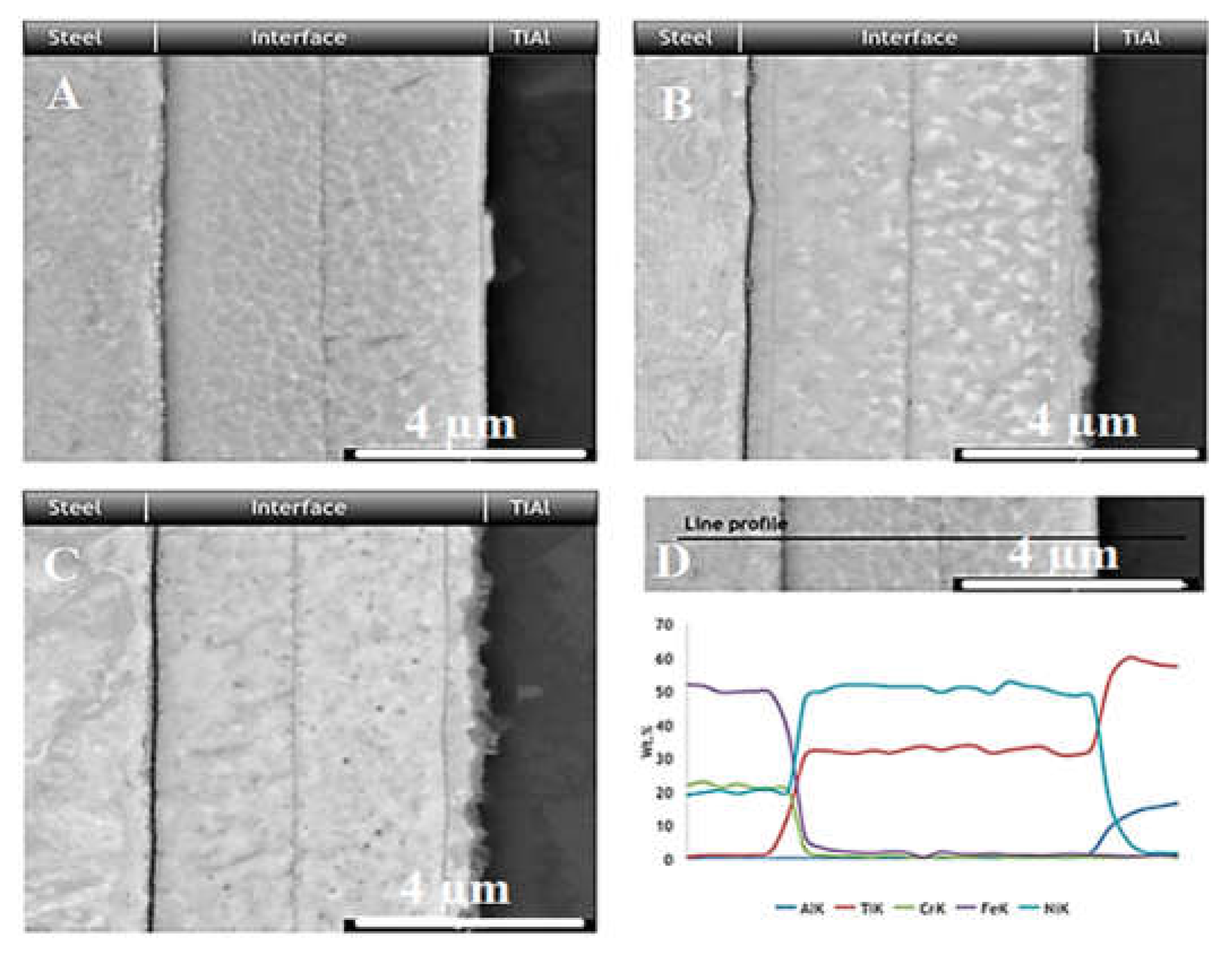
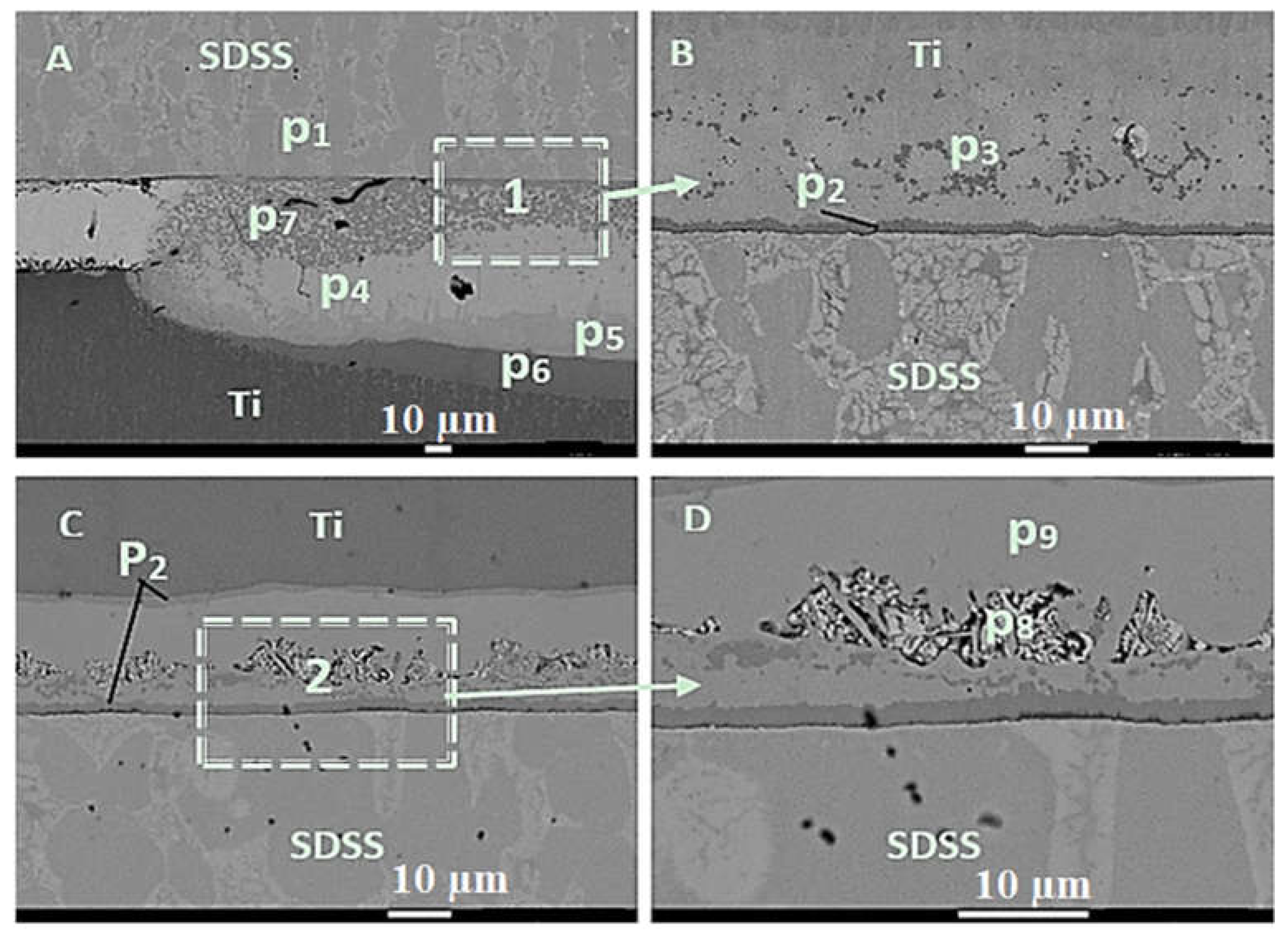
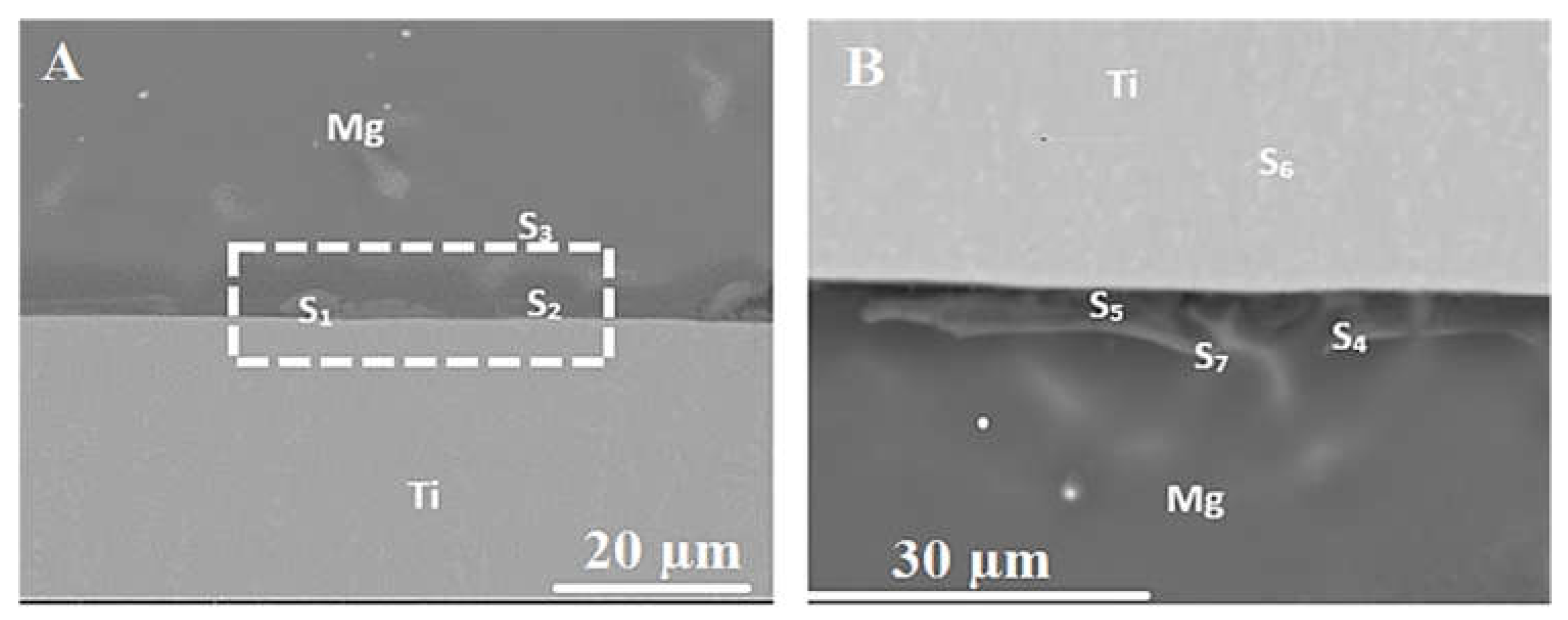
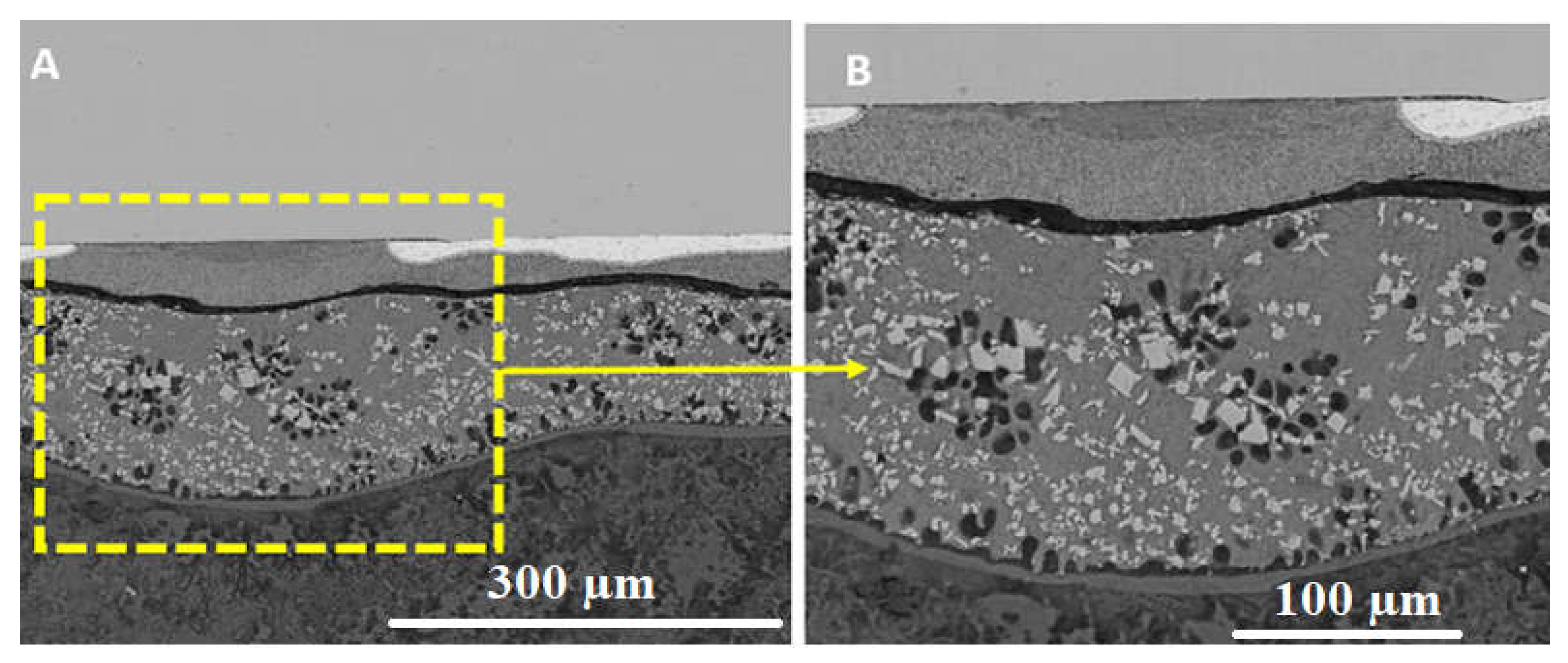
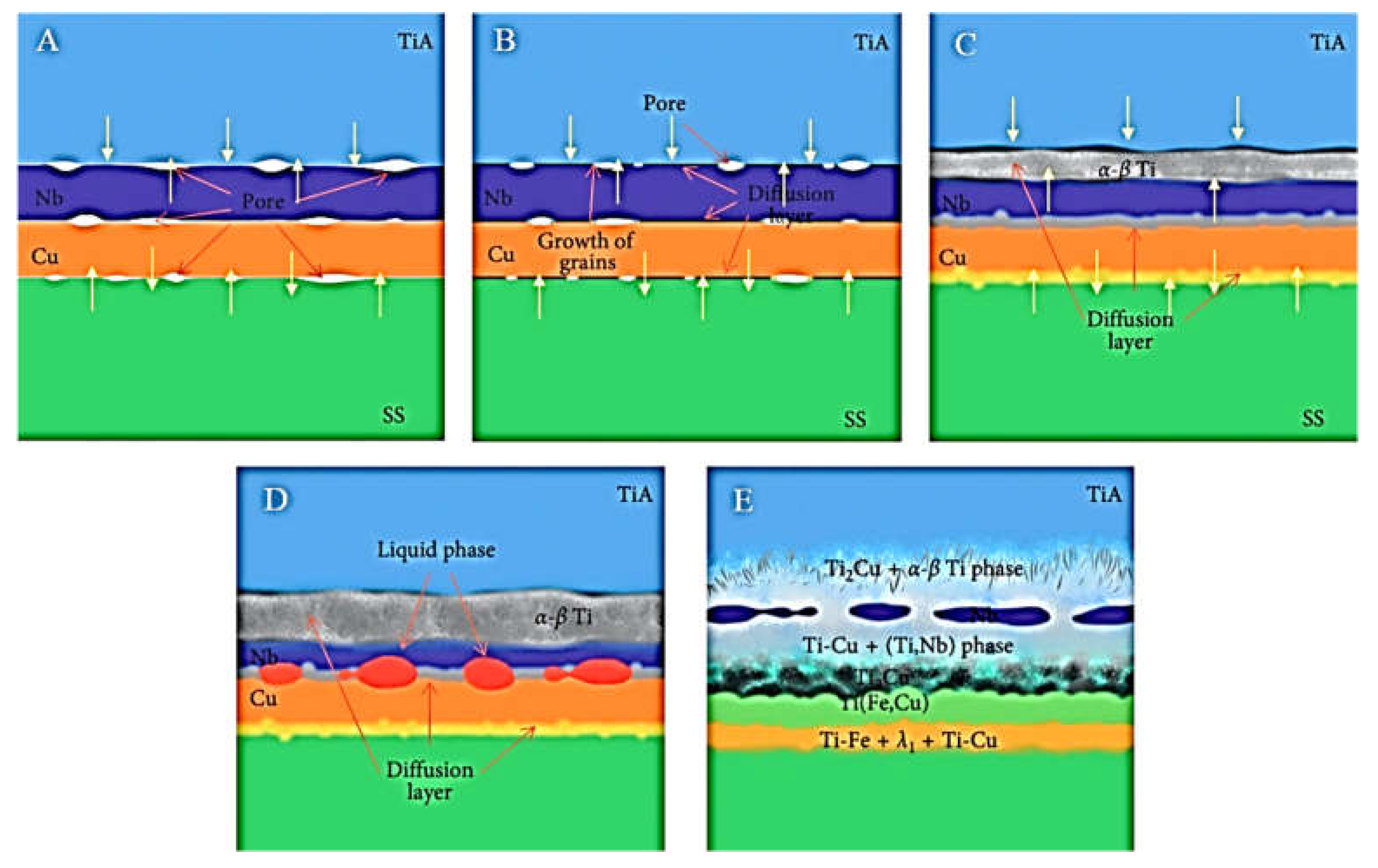
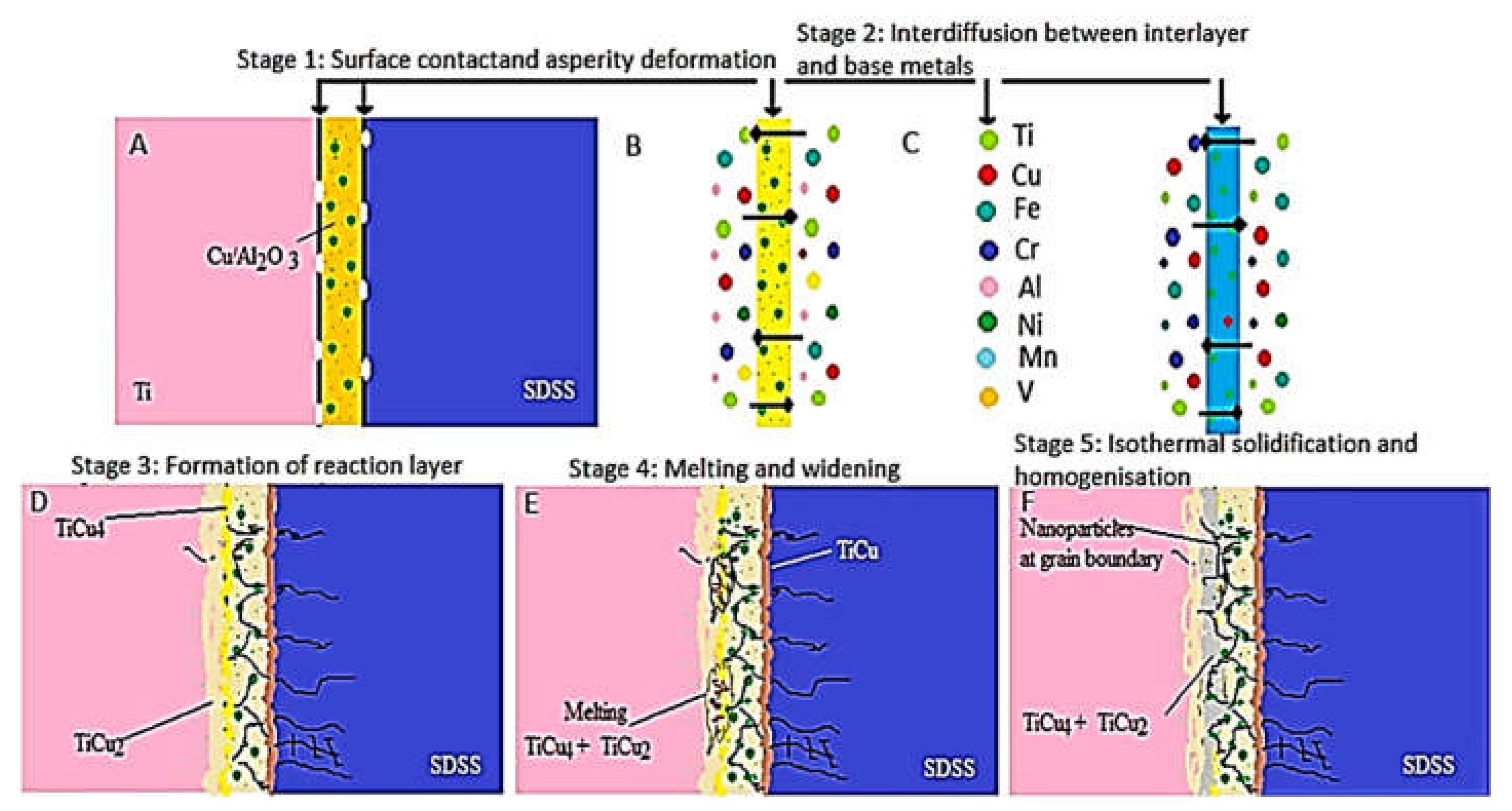
| No. | Welding /Joining Process | Parent Materials | Interlayers Materials | Strength (MPa) | Interface Hardness (HV) | Remarks | Ref. |
|---|---|---|---|---|---|---|---|
| 1 | FSW | Ti-6Al-4V to 30CrMnSiNi2A | None | 490–640 | 500–800 | Increased rotational speed resulted in less thickness of affected interlayer and strength higher than base metal alloy of medium carbon steel. | [32] |
| 2 | Pure Ti to Pure Mg | Al foil | 150 | Not available | As Al foil thickness increased, the welding defects increased due to reduced materials flow. | [33] | |
| 3 | LW | Ti-Al-V/Al-Cu-Li & Ti-Al-V/Al-Mg-Li | None | 250 100 | 350–450 350–430 | Alloying elements affected the joint properties, i.e., Cu vs. Mg such that it increased by 2.5×. Also, the laser beam offset towards Ti alloy. | [34] |
| 4 | Al-Cu-Li and Ti-Al-V | None | 103–272 | Not available | Mechanical characteristics of the intermetallic layer substantially depended on the composition of the alloying elements of the aluminium alloy. | [35] | |
| 5 | TC4 Ti alloy to 304 austenitic stainless steel | 38Zn-61Cu alloy filler | 128 | 100–420 | The laser beam at the Ti alloy side and produced a variety of intermetallic compounds at the bond interface. | [36] | |
| 6 | Ti–22Al–25Nb to TA15 | None | 943–1011 | 260–350 | O phase was formed in the fusion zone while applying dual-beam laser due to decrease of cooling rate. | [37] | |
| 7 | TC4 Titanium (Ti) alloy and SUS301 L | None | 350 | 350–450 | At the peak temp of 1116 °C, the liquid phase formed and existed only in the narrow region of interface with eutectic phase formation and β-Ti solid-solution. | [38] | |
| 8 | TC4/TA15 | None | 50–700 | 300–420 | Coarse β columnar crystals that contain acicular α’ martensitic phase inside fusion zone. | [39] | |
| 9 | Ultrasonic assisted FSW | Ti-6Al-4V to 6061-T6 aluminium | None | 236 | 60–380 | Diffusion like bonding without the intermetallic compounds observed at the joint interface. | [40] |
| 10 | RW | Ti-6Al-4V to EW140 glass fabric with PEI | Carbon nanotube lamina | 17.3 | Not available | Successful joining of Ti alloy to GF/PEI laminate. Welding time severely affected the joining process. | [41] |
| 11 | EBW | Ti55 to TA15 | None | 650–1050 | 310–380 | Formation of martensite α’ and acicular α within the fusion zone. | [42] |
| 12 | DB | Ti–6Al–4V to Mg–AZ31 | Ni foil | 5–45 | 50–420 | Bonding mechanism involves Ni–Mg eutectic formation at the Mg-alloy interface with solid-state diffusion and bond formation at the Ti-alloy interface. | [16] |
| 13 | Titanium Alloys and Stainless Steels | Not Available | 194 | Not Available | Influence of Cu, Ni (or nickel alloy), and Ag interlayers on the microstructures and mechanical properties of the joints. | [25] | |
| 14 | TLP bonding | Ti–6Al–4V to Mg–AZ31 | Ni & Cu nanoparticles | 19–69 | 50–400 | Use of Cu nanoparticles as a dispersion produced the maximum joint shear strength of 69 MPa. | [43] |
| 15 | Ti–6Al–4V to Mg–AZ31 | Ni & Cu foils | 12–55 | 50–400 | Formation of phase (Mg), (CuMg2), (Mg2Ni) and Mg3AlNi2. | [44] | |
| 16 | Ti–6Al–4V to Mg–AZ31 | Ni electro-deposited coats | 26–61 | 50–350 | Increasing the bonding temperature from 500 to 540 ◦C resulted in a change in the bonding mechanism from solid-state to eutectic liquid formation. | [15] | |
| 17 | Ti–6Al–4V to Al7075 | Cu coatings and Sn–3.6Ag–1Cu interlayers | 15–42 | 120–500 | Results showed that the Sn–3.6Ag–1Cu interlayer resulted in good joints with a thin Cu interlayer. | [45] |
| Phase | Al | Ti | V | Fe | Cu | C | Cr | Ni | Possible Phase |
|---|---|---|---|---|---|---|---|---|---|
| p1 | - | 0.22 | - | 61.57 | 0.68 | 3.51 | 24.45 | 5.73 | |
| p2 | 1.86 | 28.64 | 1.03 | 0.54 | 67.34 | - | - | 0.59 | TiCu2 |
| P3 | 2.43 | 33.78 | 1.24 | 1.26 | 58.45 | 2.34 | - | - | TiCu2 |
| P4 | 0.32 | 41.31 | 0.73 | - | 55.54 | 2.1 | - | - | TiCu |
| P5 | 1.36 | 41.55 | 1.40 | 1.13 | 51.32 | 2.89 | TiCu | ||
| P6 | 5.53 | 79.00 | 4.83 | 0.45 | 10.18 | - | - | - | Ti2Cu |
| P7 | 7.49 | 47.08 | 1.95 | 40.47 | 1.47 | 1.08 | 0.46 | TiCu-Al2O3 | |
| P8 | 1.35 | 41.55 | 1.40 | 1.13 | 51.32 | 2.89 | 0.36 | - | Tix Cux-Fex |
| P9 | 2.19 | 54.19 | 1.91 | 0.81 | 37.77 | 2.58 | 0.29 | 0.26 | Ti2Cu |
| Base Metal 1 | Interlayer Material | Basemetal-2 | Ref. | ||||
|---|---|---|---|---|---|---|---|
| Al Alloys | Mg Alloys | Stainless Steels | |||||
| Ferritic | Austenitic | Duplex | |||||
| Ti | None | N | P | G | G | F | [23,66,77,78] |
| Ti | Cu | F | F | F | G | F | [24,73,79,80,81] |
| Ti | Ni | N | F | F | F | F | [15,74,82] |
| Ti | Ag | N | N | E | G | G | [83,84] |
| Ti | Al | N | N | F | F | N | [67] |
| Ti | Ga | F | N | N | N | N | [85] |
| Ti | Cu-Nb | N | N | G | G | N | [59] |
| Ti | Nb/Cu/Ni | N | N | F | F | F | [86] |
| Ti | Cu-Zn | P | N | N | N | N | [87] |
| Ti | Cu-Ni | N | P | N | N | N | [43] |
| Ti | Ni/Al2O3 | N | N | N | N | G | [68] |
| Ti | Cu/Al2O3 | N | G | N | N | N | This work |
| Ti | V-Cr-Ni | N | N | E | E | N | [88] |
| Ti | Ni–Cr–B | N | N | N | N | F | [89] |
| Ti | Sn-3.6Ag -1Cu | P | N | N | N | N | [45] |
| Ti | Sn-10Zn-3.5Bi | P | N | N | N | N | [55] |
| Ti | Sn-4Ag-3.5Bi | P | N | N | N | N | [90] |
| Ti | Sn-5.3Ag-2Bi | F | N | N | N | N | [91] |
| Ti | Al-Si-Cu-Ge | F | N | N | N | N | [92] |
| Ti | Ni–17Cr–9Fe | N | N | N | N | G | [77] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cooke, K.O.; Atieh, A.M. Current Trends in Dissimilar Diffusion Bonding of Titanium Alloys to Stainless Steels, Aluminium and Magnesium. J. Manuf. Mater. Process. 2020, 4, 39. https://doi.org/10.3390/jmmp4020039
Cooke KO, Atieh AM. Current Trends in Dissimilar Diffusion Bonding of Titanium Alloys to Stainless Steels, Aluminium and Magnesium. Journal of Manufacturing and Materials Processing. 2020; 4(2):39. https://doi.org/10.3390/jmmp4020039
Chicago/Turabian StyleCooke, Kavian O., and Anas M. Atieh. 2020. "Current Trends in Dissimilar Diffusion Bonding of Titanium Alloys to Stainless Steels, Aluminium and Magnesium" Journal of Manufacturing and Materials Processing 4, no. 2: 39. https://doi.org/10.3390/jmmp4020039
APA StyleCooke, K. O., & Atieh, A. M. (2020). Current Trends in Dissimilar Diffusion Bonding of Titanium Alloys to Stainless Steels, Aluminium and Magnesium. Journal of Manufacturing and Materials Processing, 4(2), 39. https://doi.org/10.3390/jmmp4020039






