The Role of Substrate Surface Roughness on in-Pack Aluminization Kinetics of Ni-Base Superalloy
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Substrate Surface Roughness Description
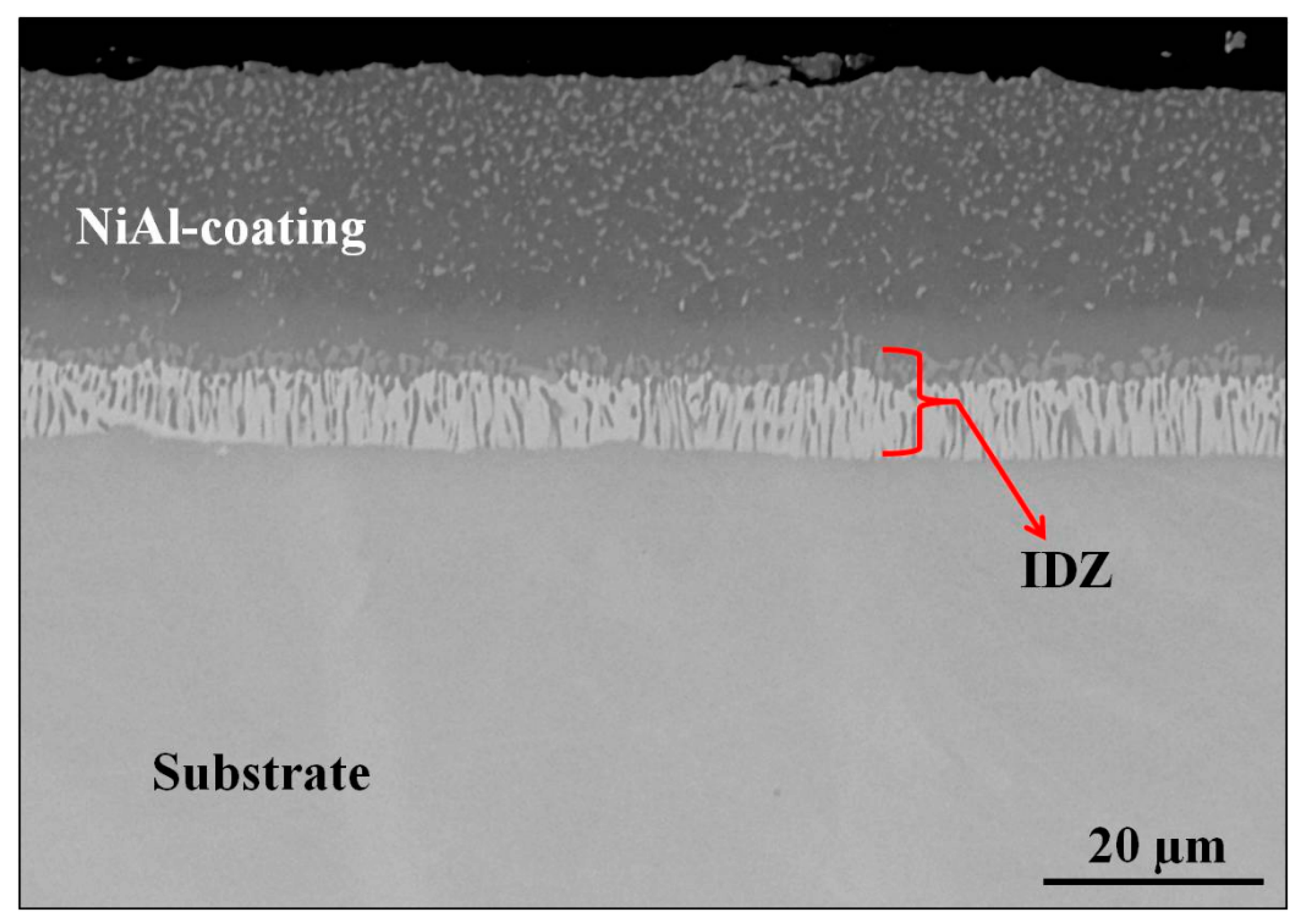
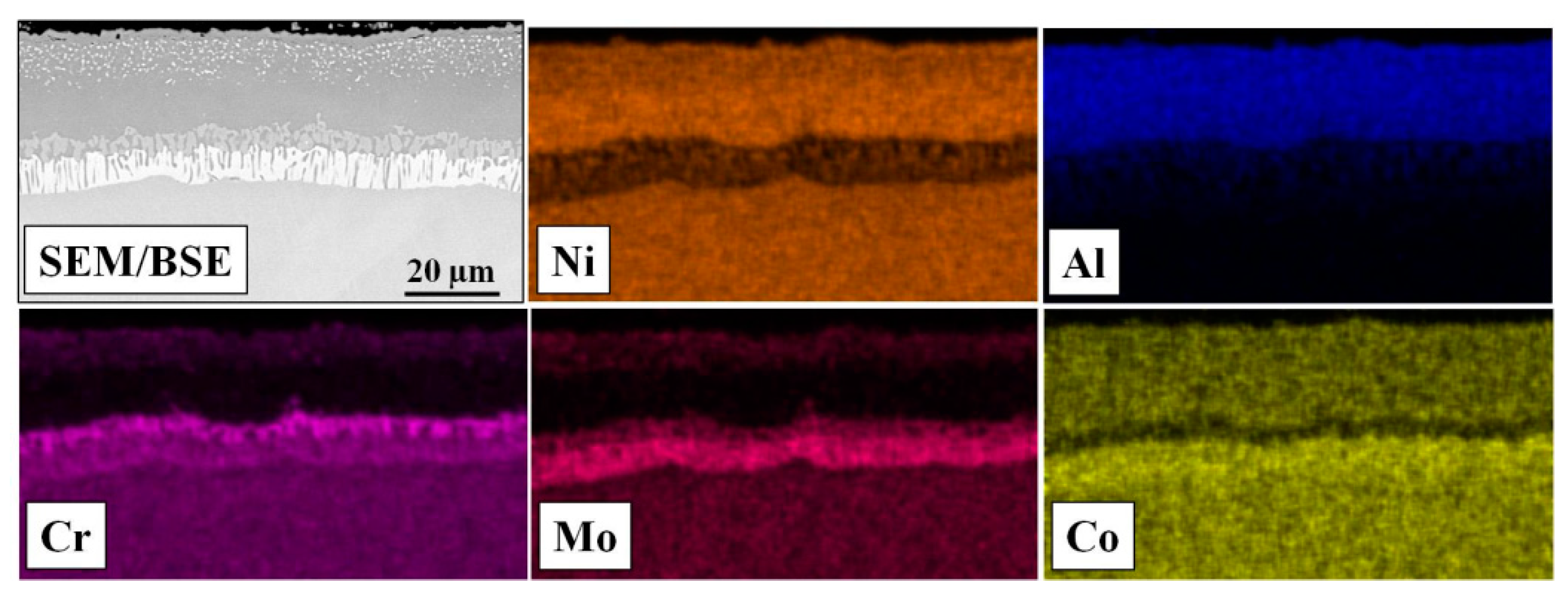
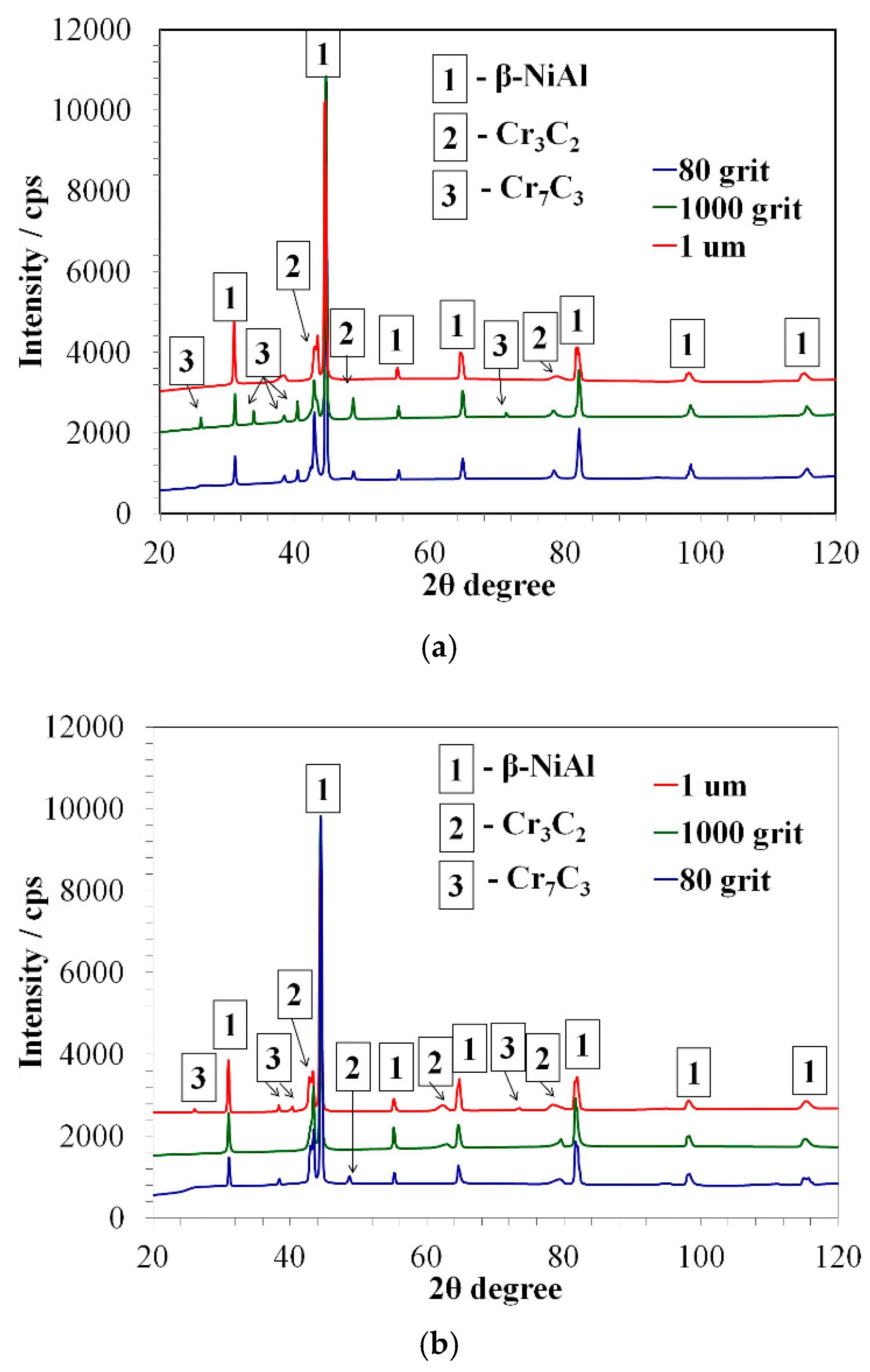
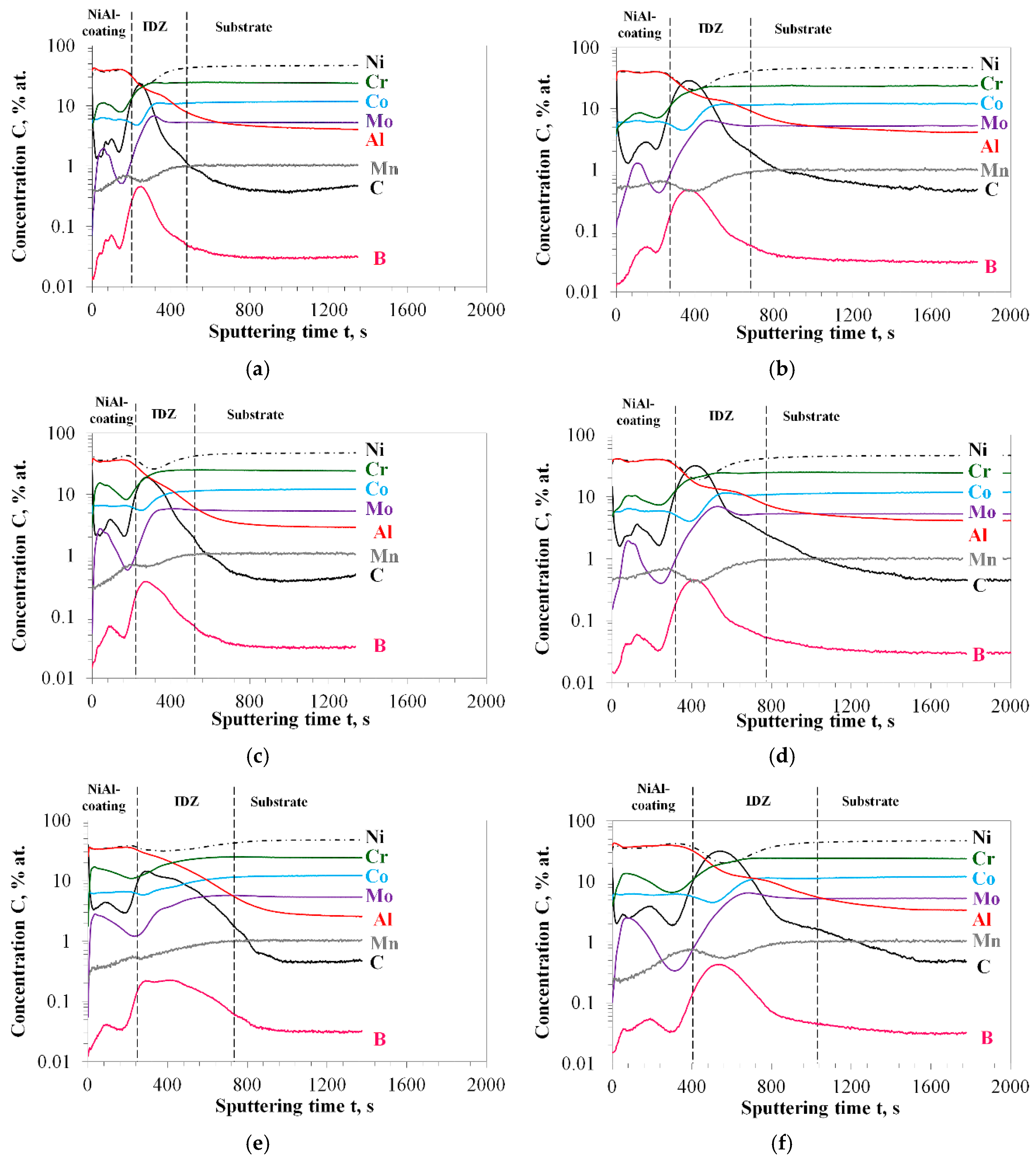
3.2. Characterization of Aluminized IN 617
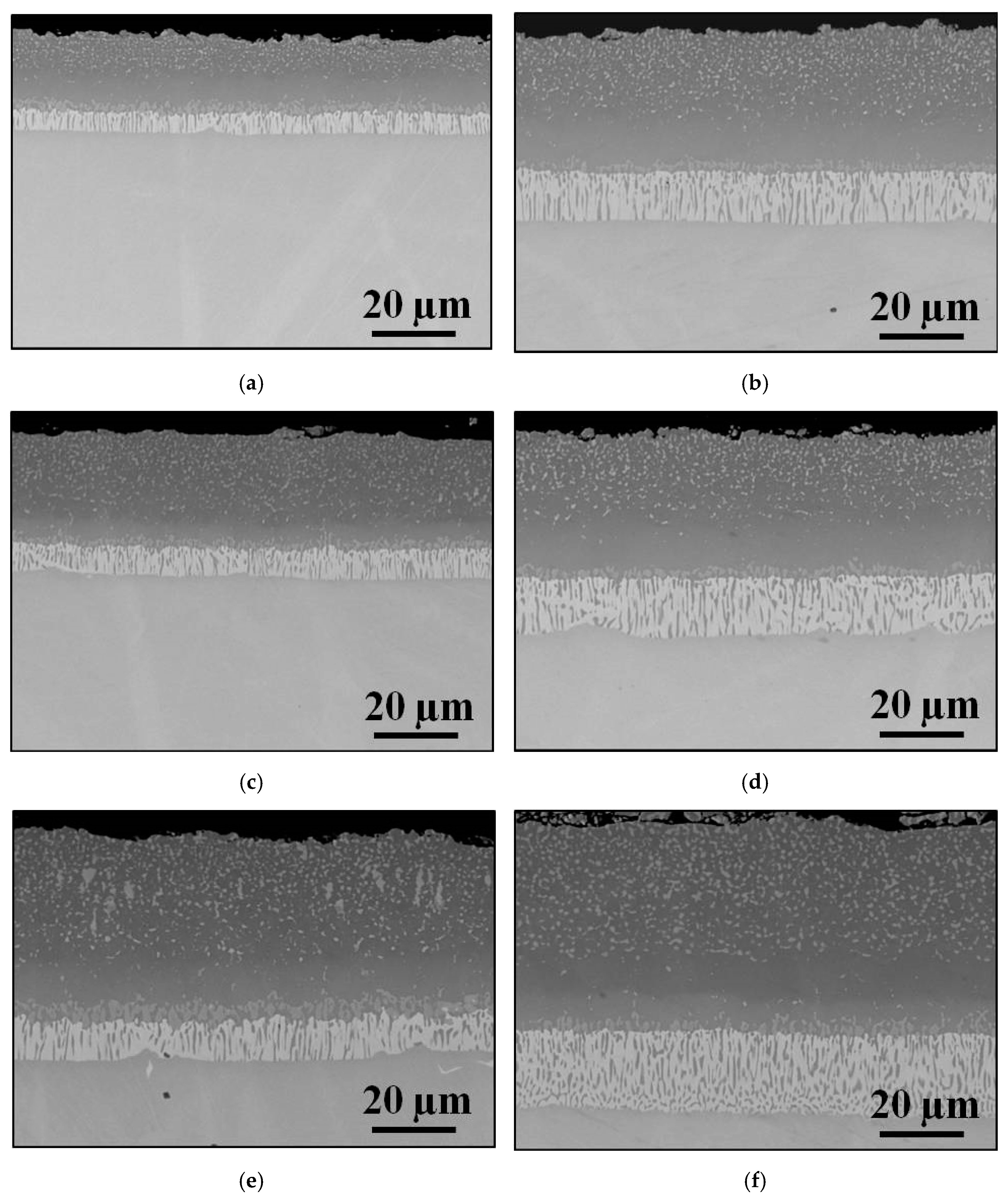
3.3. Effect of Surface Roughness on Aluminization Kinetics of IN 617
4. Discussion
5. Conclusions
- Different surface roughness did not affect the phase composition of aluminide layers and IDZ produced on the alloys with differently prepared surfaces.
- Strong influence of surface roughness on aluminization process kinetics was observed at both exposure times, namely an increase of surface-to-volume ratio (represented by SDR parameter) resulted in an increase of both: NiAl-coating and IDZ’s thicknesses.
- The dependence between the SDR parameter and the measured thicknesses was found to be logarithmic. The slope of the fitting curves was different for different measured zones and exposure times.
- Aluminization kinetics is much more influenced by a surface preparation method at early stages of the process rather than later.
Author Contributions
Funding
Conflicts of Interest
References
- Giggins, C.S.; Pettit, F.S. Oxidation of Ni-Cr-Al Alloys Between 1100 °C and 1200 °C. Solid State Sci. 1971, 118, 1782–1790. [Google Scholar]
- Danielewski, M.; Grzesik, Z.; Mrowec, S. On the oxidation mechanism of Ni–Pt alloys at high temperatures. Corros. Sci. 2011, 53, 2785–2792. [Google Scholar]
- Holcomb, G.R. Calculation of Reactive-evaporation Rates of Chromia. Oxid. Met. 2008, 69, 163–180. [Google Scholar] [CrossRef]
- Birks, N.; Meier, G.H.; Petit, F.S. Introduction to the High- Temperature Oxidation of Metal; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar]
- Nicholls, J.R.; Simms, N.J. Shreir’s Corrosion; Richardson, T., Ed.; Elsevier: Amsterdam, NL, USA, 2010; Volume 1, pp. 518–540. [Google Scholar]
- Darolia, R. Thermal barrier coatings technology: Critical review, progress update, remaining challenges and prospects. Int. Mater. Rev. 2013, 58, 315–348. [Google Scholar] [CrossRef]
- Nowak, W.; Naumenko, D.; Mor, G.; Mor, F.; Mack, D.E.; Vassen, R.; Singheiser, L.; Quadakkers, W.J. Effect of processing parameters on MCrAlY bondcoat roughness and lifetime of APS-TBC systems. Surf. Coat. Technol. 2014, 260, 82–89. [Google Scholar] [CrossRef]
- Hejrani, E.; Sebold, D.; Nowak, W.J.; Mauer, G.; Naumenko, D.; Vassen, R.; Quadakkers, W.J. Isothermal and cyclic oxidation behavior of free standing MCrAlY coatings manufactured by high-velocity atmospheric plasma spraying. Surf. Coat. Technol. 2017, 313, 191–201. [Google Scholar] [CrossRef]
- Nowak, W.J.; Kubaszek, T.; Góral, M.; Wierzba, B. Durability of underaluminized thermal barrier coatings during exposure at high temperature. Surf. Coat. Technol. 2020, 382, 125236. [Google Scholar] [CrossRef]
- Zielińska, M.; Zagula-Yaworska, M.; Sieniawski, J.; Filip, R. Microstructure and oxidation resistance of an aluminide coating on the nickel based superalloy MAR M247 deposited by the CVD aluminizing process. Arch. Metall. Mater. 2013, 58, 697–701. [Google Scholar] [CrossRef]
- Rannou, B.; Bouchaud, B.; Balmain, J.; Bonnet, G.; Pedraza, F. Comparative Isothermal Oxidation Behaviour of New Aluminide Coatings from Slurries Containing Al Particles and Conventional Out-of-Pack Aluminide Coatings. Oxid. Met. 2014, 81, 139–149. [Google Scholar] [CrossRef]
- Rannou, B.; Velasco, F.; Guzmán, C.; Kolarik, V.; Pedraza, F. Aging and thermal behavior of a PVA/Al microspheres slurry for aluminizing purposes. Mater. Chem. Phys. 2012, 134, 360–365. [Google Scholar] [CrossRef]
- Romanowska, J.; Zagula-Yavorska, M.; Sieniawski, J.; Markowski, J. Zirconium Modified Aluminide Coatings Obtained by the CVD and PVD Methods. Open J. Met. 2013, 3, 92–99. [Google Scholar] [CrossRef][Green Version]
- Dehghanghadikolaei, A.; Ibrahim, H.; Amerinatanzi, A.; Hashemi, M.; Moghaddam, N.S.; Elahinia, M. Improving corrosion resistance of additively manufactured nickel–titanium biomedical devices by micro-arc oxidation process. J. Mater. Sci. 2019, 54, 7333–7355. [Google Scholar] [CrossRef]
- Dehghanghadikolaei, A.; Fotovvati, B. Coating Techniques for Functional Enhancement of Metal Implants for Bone Replacement: A Review. Materials 2019, 12, 1795. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Huang, X.; Liu, R.; Yang, Q. A Study of Pack Aluminizing Process for NiCrAlY Coatings Using Response Surface Methodology. J. Mater. Eng. Perform. 2014, 23, 83–91. [Google Scholar] [CrossRef]
- Bozza, F.; Bolelli, G.; Giolli, C.; Giorgetti, A.; Lusvarghi, L.; Sassatelli, P.; Scrivani, A.; Candeli, A.; Thoma, M. Diffusion mechanisms and microstructure development in pack aluminizing of Ni-based alloys. Surf. Coat. Technol. 2014, 239, 147–159. [Google Scholar] [CrossRef]
- Nowotnik, A.; Sieniawski, J.; Góral, M.; Pytel, M.; Dychtoń, K. Microstructure and kinetic growth of aluminide coatings deposited by the CVD method on Re 80 superalloy. Arch. Mater. Sci. Eng. 2012, 55, 22–28. [Google Scholar]
- Nowotnik, A.; Góral, M.; Pytel, M.; Dychtoń, K. Influence of coatings deposition parameters on microstructure of aluminide coatings deposited by CVD method on Ni-superalloys. Sol. St. Phen. 2013, 197, 95–100. [Google Scholar] [CrossRef]
- Kochmańska, A.; Kochmański, P.; Kawiak, M. The structure of aluminide coatings on alloy steels in the area of the welded joints. Inż. Mater. 2017, 3, 131–135. [Google Scholar]
- Kochmańska, A.E. Aluminide coatings on Inconel 617 obtained by slurry method with inorganic binder. J. Achiev. Mater. Manuf. Eng. 2017, 85, 49–55. [Google Scholar] [CrossRef]
- Nowak, W.J.; Ochał, K.; Wierzba, P.; Gancarczyk, K.; Wierzba, B. Effect of Substrate Roughness on Oxidation Resistance of an Aluminized Ni-Base Superalloy. Metals 2019, 9, 782–795. [Google Scholar] [CrossRef]
- Serafin, D.; Nowak, W.J.; Wierzba, B. The effect of surface preparation on high temperature oxidation of Ni, Cu and Ni-Cu alloy. Appl. Surf. Sci. 2019, 476, 442–451. [Google Scholar] [CrossRef]
- Sreenath, S.; Shivakumar, M.R. Evaluation of Surface Finish and its Effect on Packed Diffusion Aluminisation of Low Alloy Steel Component. Int. J. Appl. Innov. Eng. Man. 2018, 7, 77–83. [Google Scholar]
- Dong, J.; Sun, Y.; He, F.; Huang, H.; Zhen, J. Effects of substrate surface roughness and a luminizing agent composition on the aluminide coating coatings by low-temperature pack cementation. Mater. Res. Express 2018, 6, 036409. [Google Scholar] [CrossRef]
- Pfeifer, J.P.; Holzbrecher, H.; Quadakkers, W.J.; Speier, J. Quantitative analysis of oxide films on ODS-alloys using MCs+-SIMS and e-beam SNMS. J. Anal. Chem. 1993, 346, 186–191. [Google Scholar] [CrossRef]
- Quadakkers, W.J.; Elschner, A.; Speier, W.; Nickel, H. Composition and growth mechanisms of alumina scales on FeCrAl-based alloys determined by SNMS. Appl. Surf. Sci. 1991, 52, 271–287. [Google Scholar] [CrossRef]
- Nowak, W.J. Characterization of oxidized Ni-based superalloys by GD-OES. J. Anal. At. Spectrom. 2017, 32, 1730–1738. [Google Scholar] [CrossRef]
- Nowak, W.J.; Serafin, D.; Wierzba, B. Effect of surface mechanical treatment on the oxidation behavior of FeAl-model alloy. J. Mater. Sci. 2019, 54, 9185–9196. [Google Scholar] [CrossRef]


| Parameter | Surface Preparation Method | ||
|---|---|---|---|
| Polishing (1 µm) | Grinding (1000 Grit) | Grinding (80 Grit) | |
| Ra (µm) | 0.00949 | 0.0113 | 0.473 |
| SDR (%) | 0.000355 | 0.000943 | 3.23 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, W.J.; Tomków, M.; Wierzba, P.; Gancarczyk, K.; Wierzba, B. The Role of Substrate Surface Roughness on in-Pack Aluminization Kinetics of Ni-Base Superalloy. J. Manuf. Mater. Process. 2020, 4, 15. https://doi.org/10.3390/jmmp4010015
Nowak WJ, Tomków M, Wierzba P, Gancarczyk K, Wierzba B. The Role of Substrate Surface Roughness on in-Pack Aluminization Kinetics of Ni-Base Superalloy. Journal of Manufacturing and Materials Processing. 2020; 4(1):15. https://doi.org/10.3390/jmmp4010015
Chicago/Turabian StyleNowak, Wojciech J., Małgorzata Tomków, Patrycja Wierzba, Kamil Gancarczyk, and Bartek Wierzba. 2020. "The Role of Substrate Surface Roughness on in-Pack Aluminization Kinetics of Ni-Base Superalloy" Journal of Manufacturing and Materials Processing 4, no. 1: 15. https://doi.org/10.3390/jmmp4010015
APA StyleNowak, W. J., Tomków, M., Wierzba, P., Gancarczyk, K., & Wierzba, B. (2020). The Role of Substrate Surface Roughness on in-Pack Aluminization Kinetics of Ni-Base Superalloy. Journal of Manufacturing and Materials Processing, 4(1), 15. https://doi.org/10.3390/jmmp4010015






