The Behavioral Responses of Geoffroy’s Spider Monkeys to Drone Flights
Abstract
1. Introduction
2. Materials and Methods
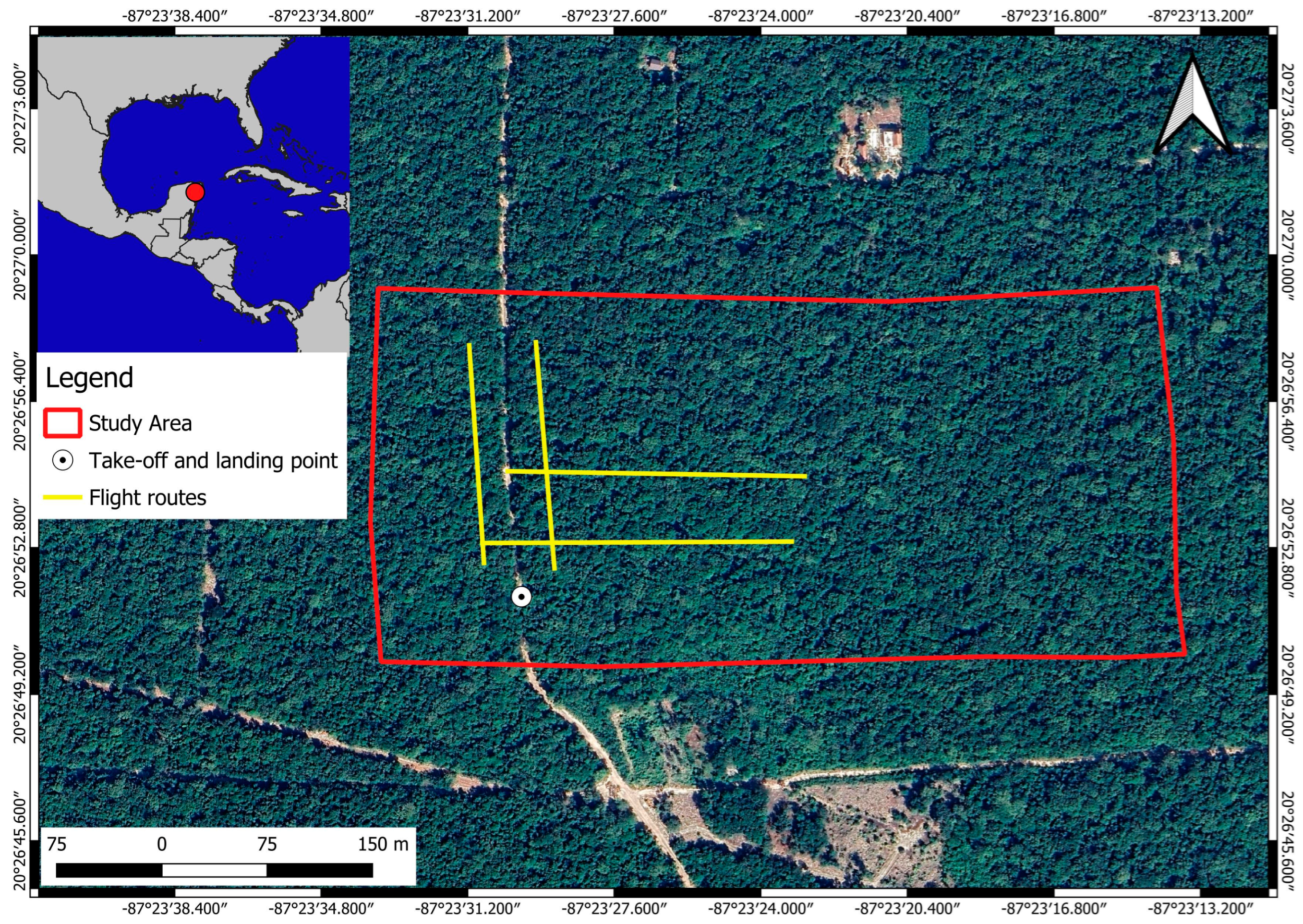
2.1. Study Site
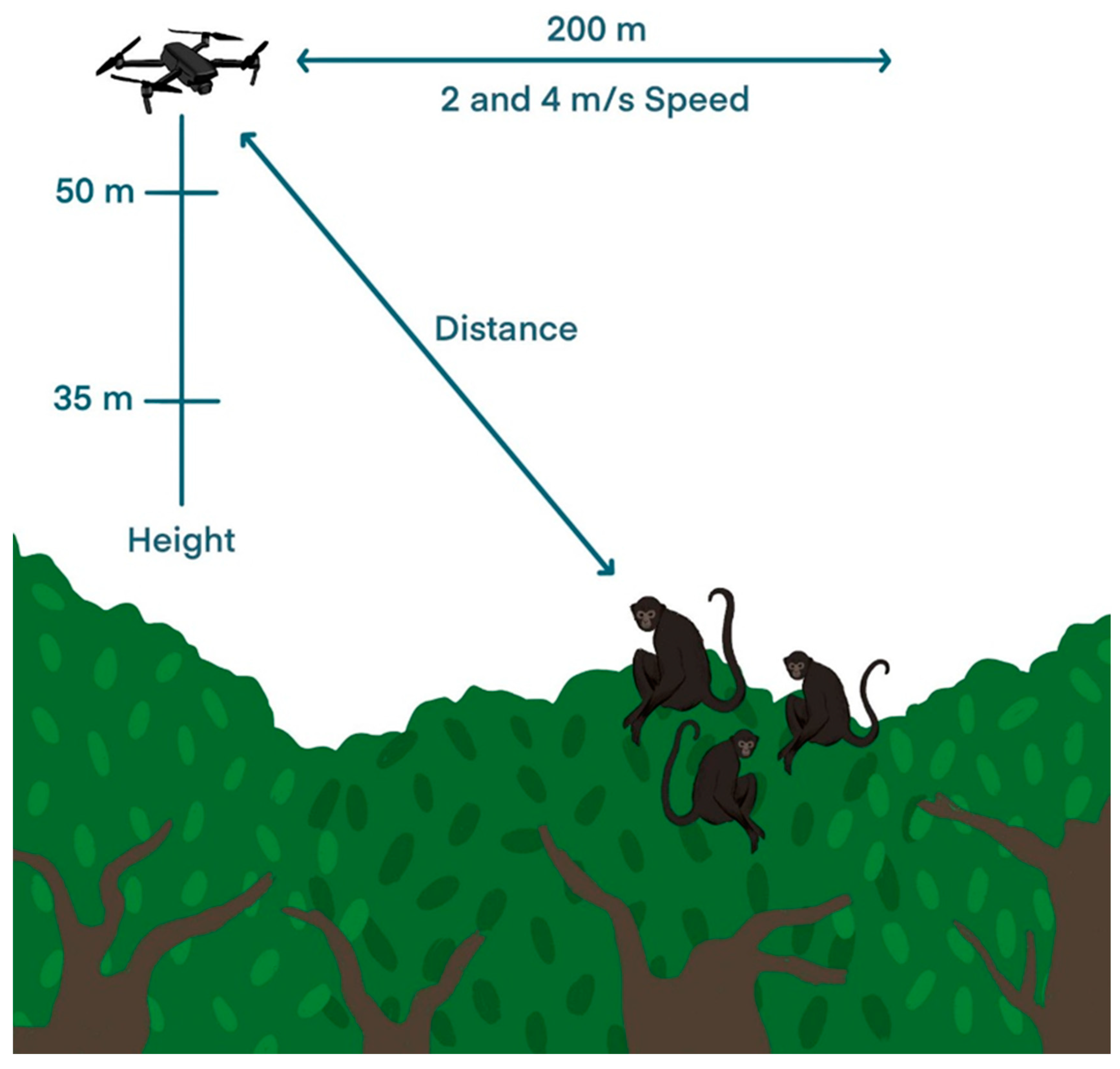
2.2. Study Design
Flight Parameters
2.3. Data Collection
2.4. Data Analysis
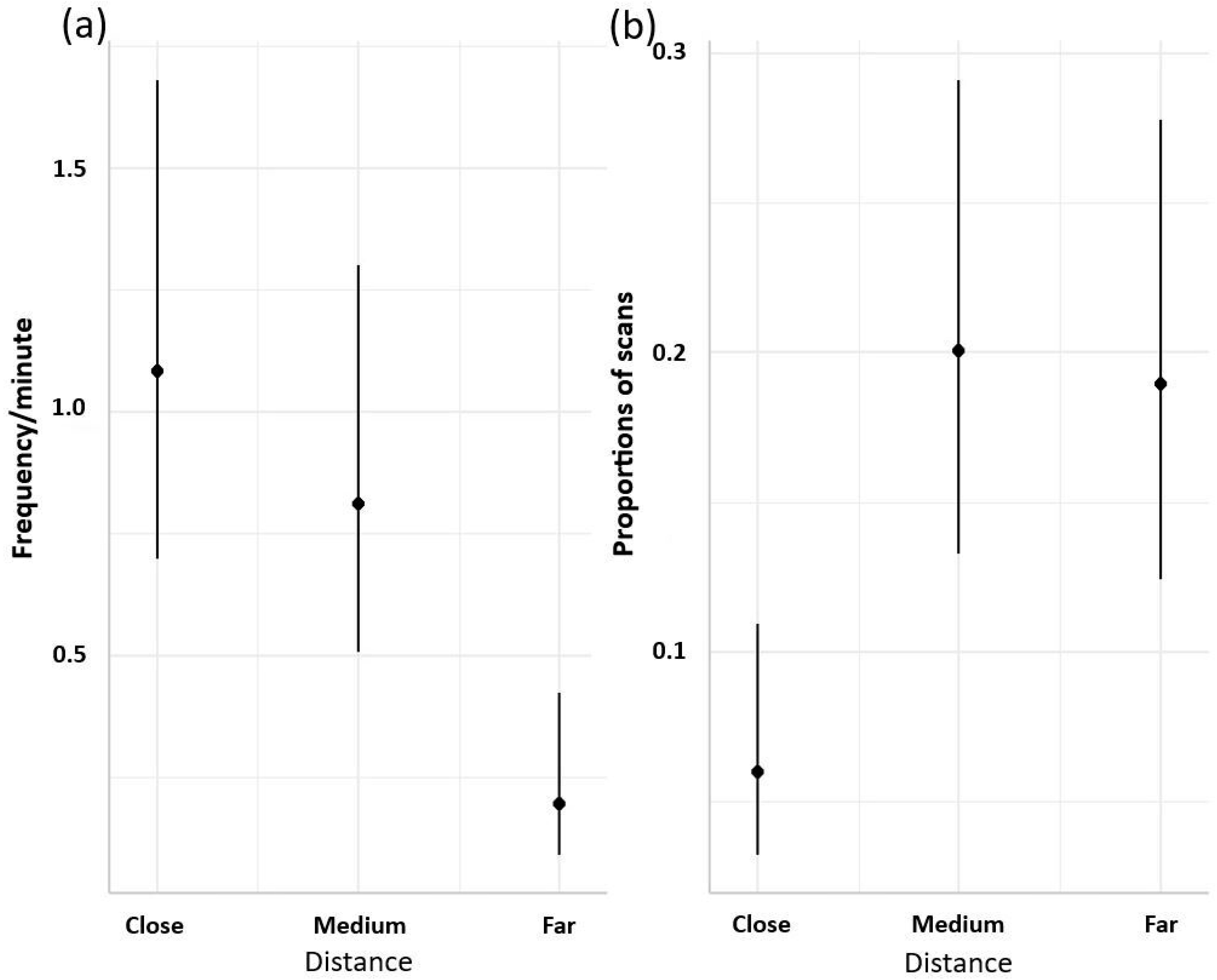
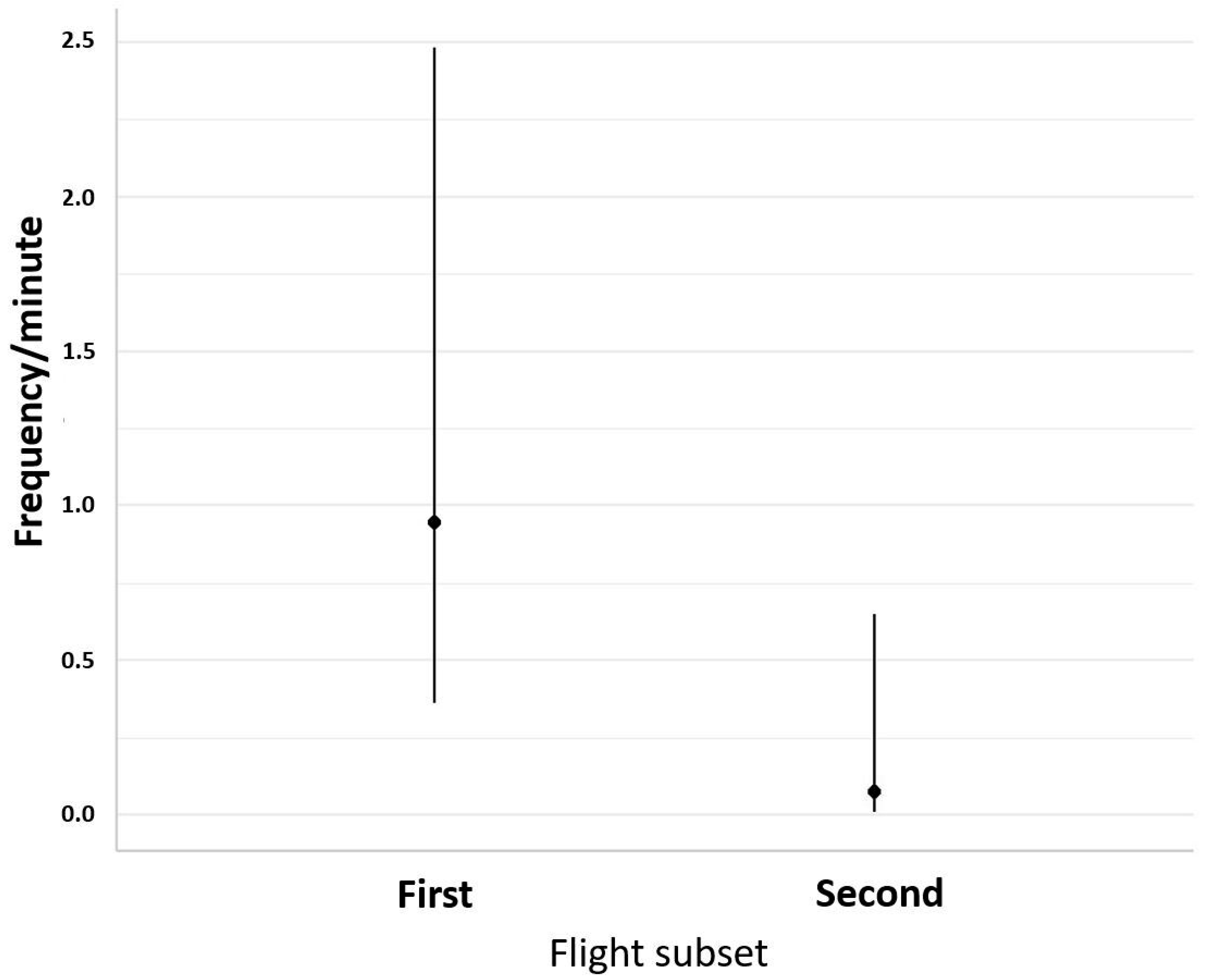
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sandbrook, C. The social implications of using drones for biodiversity conservation. Ambio 2015, 44, 636–647. [Google Scholar] [CrossRef] [PubMed]
- Ivosevic, B.Y.-G.Y.O. The use of conservation drones in ecology and wildlife research. J. Ecol. Environ. 2015, 38, 113–118. [Google Scholar] [CrossRef]
- López, J.J.; Mulero-Pázmány, M. Drones for conservation in protected areas: Present and future. Drones 2019, 3, 10. [Google Scholar] [CrossRef]
- Orange, J.P.; Bielefeld, R.R.; Cox, W.A.; Sylvia, A.L. Impacts of drone flight altitude on behaviors and species identification of Marsh birds in Florida. Drones 2023, 7, 584. [Google Scholar] [CrossRef]
- Koger, B.; Deshpande, A.; Kerby, J.T.; Graving, J.M.; Costelloe, B.R.; Couzin, I.D. Quantifying the movement, behaviour and environmental context of group-living animals using drones and computer vision. J. Anim. Ecol. 2023, 92, 1357–1371. [Google Scholar] [CrossRef]
- Brack, I.V.; Kindel, A.; Oliveira, L.F.B. Detection errors in wildlife abundance estimates from Unmanned Aerial Systems (UAS) surveys: Synthesis, solutions, and challenges. Methods Ecol. Evol. 2018, 9, 1864–1873. [Google Scholar] [CrossRef]
- Brack, I.V.; Kindel, A.; de Oliveira, L.F.B.; Lahoz-Monfort, J.J. Optimally designing drone-based surveys for wildlife abundance estimation with N-mixture models. Methods Ecol. Evol. 2023, 14, 898–910. [Google Scholar] [CrossRef]
- McCarthy, E.D.; Martin, J.M.; Boer, M.M.; Welbergen, J.A. Drone-based thermal remote sensing provides an effective new tool for monitoring the abundance of roosting fruit bats. Remote Sens. Ecol. Conserv. 2021, 7, 461–474. [Google Scholar] [CrossRef]
- Bevan, E.; Whiting, S.; Tucker, T.; Guinea, M.; Raith, A.; Douglas, R. Measuring behavioral responses of sea turtles, saltwater crocodiles, and crested terns to drone disturbance to define ethical operating thresholds. PLoS ONE 2018, 13, e0194460. [Google Scholar] [CrossRef]
- Linchant, J.; Lisein, J.; Semeki, J.; Lejeune, P.; Vermeulen, C. Are unmanned aircraft systems (UASs) the future of wildlife monitoring? A review of accomplishments and challenges. Mamm. Rev. 2015, 45, 239–252. [Google Scholar] [CrossRef]
- Mulero-Pázmány, M.; Jenni-Eiermann, S.; Strebel, N.; Sattler, T.; Negro, J.J.; Tablado, Z. Unmanned aircraft systems as a new source of disturbance for wildlife: A systematic review. PLoS ONE 2017, 12, e0178448. [Google Scholar] [CrossRef] [PubMed]
- Lima, S.L.; Dill, L.M. Behavioral decisions made under the risk of predation: A review and prospectus. Can. J. Zool. 1990, 68, 619–640. [Google Scholar] [CrossRef]
- Headland, T.; Ostendorf, B.; Taggart, D. The behavioral responses of a nocturnal burrowing marsupial (Lasiorhinus latifrons) to drone flight. Ecol. Evol. 2021, 11, 12173–12181. [Google Scholar] [CrossRef] [PubMed]
- Kavaliers, M.; Choleris, E. Antipredator responses and defensive behavior: Ecological and ethological approaches for the neurosciences. Neurosci. Biobehav. Rev. 2001, 25, 577–586. [Google Scholar] [CrossRef]
- Weston, M.A.; O’Brien, C.; Kostoglou, K.N.; Symonds, M.R.E. Escape responses of terrestrial and aquatic birds to drones: Towards a code of practice to minimize disturbance. J. Appl. Ecol. 2020, 57, 777–785. [Google Scholar] [CrossRef]
- Frixione, M.G.; Salvadeo, C. Drones, Gulls and Urbanity: Interaction between New Technologies and Human Subsidized Species in Coastal Areas. Drones 2021, 5, 30. [Google Scholar] [CrossRef]
- Mayer, M.; Furuhovde, E.; Nordli, K.; Myriam Ausilio, G.; Wabakken, P.; Eriksen, A.; Evans, A.L.; Mathisen, K.M.; Zimmermann, B. Monitoring GPS-collared moose by ground versus drone approaches: Efficiency and disturbance effects. Wildl. Biol. 2024, e01213. [Google Scholar] [CrossRef]
- Laundré, J.W.; Hernández, L.; Altendorf, K.B. Wolves, elk, and bison: Reestablishing the “landscape of fear” in Yellowstone National Park, U.S.A. Can. J. Zool. 2001, 79, 1401–1409. [Google Scholar] [CrossRef]
- Laundré, J.W.; Hernández, L.; Ripple, W.J. The Landscape of Fear: Ecological implications of being afraid. Open Ecol. J. 2010, 3, 1–7. [Google Scholar] [CrossRef]
- Gaynor, K.M.; Brown, J.S.; Middleton, A.D.; Power, M.E.; Brashares, J.S. Landscapes of Fear: Spatial patterns of risk perception and response. Trends Ecol. Evol. 2019, 34, 355–368. [Google Scholar] [CrossRef]
- Rebolo-Ifrán, N.; Graña Grilli, M.; Lambertucci, S.A. Drones as a Threat to Wildlife: YouTube Complements Science in Providing Evidence about Their Effect. Environ. Conserv. 2019, 46, 205–210. [Google Scholar] [CrossRef]
- Barnas, A.; Newman, R.; Felege, C.J.; Corcoran, M.P.; Hervey, S.D.; Stechmann, T.J.; Rockwell, R.F.; Ellis-Felege, S.N. Evaluating behavioral responses of nesting lesser snow geese to unmanned aircraft surveys. Ecol. Evol. 2018, 8, 1328–1338. [Google Scholar] [CrossRef] [PubMed]
- Arona, L.; Dale, J.; Heaslip, S.G.; Hammill, M.O.; Johnston, D.W. Assessing the disturbance potential of small unoccupied aircraft systems (UAS) on gray seals (Halichoerus grypus) at breeding colonies in Nova Scotia, Canada. PeerJ 2018, 6, e4467. [Google Scholar] [CrossRef] [PubMed]
- Brunton, E.; Bolin, J.; Leon, J.; Burnett, S. Fright or flight? Behavioural responses of kangaroos to drone-based monitoring. Drones 2019, 3, 41. [Google Scholar] [CrossRef]
- Colombelli-Négrel, D.; Sach, I.Z.; Hough, I.; Hodgson, J.C.; Daniels, C.B.; Kleindorfer, S. Koalas showed limited behavioural response and no physiological response to drones. Appl. Anim. Behav. Sci. 2023, 264, 105963. [Google Scholar] [CrossRef]
- Schroeder, N.M.; Panebianco, A. Sociability strongly affects the behavioural responses of wild guanacos to drones. Sci. Rep. 2021, 11, 20901. [Google Scholar] [CrossRef]
- Wegdell, F.; Hammerschmidt, K.; Fischer, J. Conserved alarm calls but rapid auditory learning in monkey responses to novel flying objects. Nat. Ecol. Evol. 2019, 3, 1039–1042. [Google Scholar] [CrossRef]
- Semel, B.P.; Karpanty, S.M.; Vololonirina, F.F.; Rakotonanahary, A.N. Eyes in the sky: Assessing the feasibility of low-cost, ready-to-use unmanned aerial vehicles to monitor primate populations directly. Folia Primatol. 2020, 91, 69–82. [Google Scholar] [CrossRef]
- Rahman, D.A.; Sitorus, A.B.Y.; Condro, A.A. From coastal to montane forest ecosystems, using drones for multi-species research in the tropics. Drones 2022, 6, 6. [Google Scholar] [CrossRef]
- Maréchal, L.; MacLarnon, A.; Majolo, B.; Semple, S. Primates’ behavioural responses to tourists: Evidence for a trade-off between potential risks and benefits. Sci. Rep. 2016, 6, 32465. [Google Scholar] [CrossRef]
- Christie, K.S.; Gilbert, S.L.; Brown, C.L.; Hatfield, M.; Hanson, L. Unmanned aircraft systems in wildlife research: Current and future applications of a transformative technology. Front. Ecol. Environ. 2016, 14, 241–251. [Google Scholar] [CrossRef]
- Whitworth, A.; Pinto, C.; Ortiz, J.; Flatt, E.; Silman, M. Flight speed and time of day heavily influence rainforest canopy wildlife counts from drone-mounted thermal camera surveys. Biodivers. Conserv. 2022, 31, 3179–3195. [Google Scholar] [CrossRef]
- Mesquita, G.P.; Mulero-Pázmány, M.; Wich, S.A.; Rodríguez-Teijeiro, J.D. Terrestrial Megafauna Response to Drone Noise Levels in Ex Situ Areas. Drones 2022, 6, 333. [Google Scholar] [CrossRef]
- Bennitt, E.; Bartlam-Brooks, H.L.A.; Hubel, T.Y.; Wilson, A.M. Terrestrial mammalian wildlife responses to Unmanned Aerial Systems approaches. Sci. Rep. 2019, 9, 2142. [Google Scholar] [CrossRef]
- Schroeder, N.M.; Panebianco, A.; Musso, R.G.; Carmanchahi, P. An experimental approach to evaluate the potential of drones in terrestrial mammal research: A gregarious ungulate as a study model. R. Soc. Open Sci. 2020, 7, 191482. [Google Scholar] [CrossRef]
- Nisbet, I.C.T. Waterbird Society disturbance, habituation, and management of waterbird colonies. Waterbirds Int. J. Waterbird Biol. 2000, 23, 312–332. [Google Scholar]
- Rahman, D.A.; Setiawan, Y.; Rahman, A.A.A.F.; Martiyani, T.R. Javan langur responses to the repeated exposure of ground survey and novel stimulus, unmanned aerial vehicles. IOP Conf. Ser. Earth Environ. Sci. 2021, 948, 012006. [Google Scholar] [CrossRef]
- Ditmer, M.A.; Werden, L.K.; Tanner, J.C.; Vincent, J.B.; Callahan, P.; Iaizzo, P.A.; Laske, T.G.; Garshelis, D.L. Bears habituate to the repeated exposure of a novel stimulus, unmanned aircraft systems. Conserv. Physiol. 2019, 7, coy067. [Google Scholar] [CrossRef]
- Van Vuuren, M.; van Vuuren, R.; Silverberg, L.M.; Manning, J.; Pacifici, K.; Dorgeloh, W.; Campbell, J. Ungulate responses and habituation to unmanned aerial vehicles in Africa’s savanna. PLoS ONE 2023, 18, e0288975. [Google Scholar] [CrossRef]
- Spaan, D.; Di Fiore, A.; Rangel-Rivera, C.E.; Hutschenreiter, A.; Wich, S.; Aureli, F. Detecting spider monkeys from the sky using a high-definition RGB camera: A rapid-assessment survey method? Biodivers. Conserv. 2022, 31, 479–496. [Google Scholar] [CrossRef]
- Aureli, F.; Schaffner, C.M.; Boesch, C.; Bearder, S.K.; Call, J.; Chapman, C.A.; Connor, R.; Di Fiore, A.; Dunbar, R.I.M.; Peter Henzi, S.; et al. Fission-fusion dynamics new research frameworks. Curr. Anthropol. 2008, 49, 627–654. [Google Scholar] [CrossRef]
- Cortes-Ortíz, L.; Solano-Rojas, D.; Rosales-Meda, M.; Williams-Guillén, K.; Méndez-Carvajal, P.G.; Marsh, L.K.; Canales-Espinosa, D.; Mittermeier, R.A. Ateles Geoffroyi (Geoffroy’s Spider Monkey), The IUCN Red List of Threatened Species 2021. Available online: https://www.iucnredlist.org/species/2279/191688782 (accessed on 1 June 2024).
- Mendez-Carvajal, P.G.; Rodríguez, M.E.; Pozo-Montuy, G.; Chaves, Ó.M.; Sánchez-Porras, R.; Gutíerrez-Pineda, K.; Spaan, D.; Pinel-Ramos, E.J.; Zaldaña-Orantes, K. Geoffroy’s spider monkey Ateles geoffroyi Kühl, 1820. In Primates in Peril: The World’s 25 Most Endangered Primates 2022–2023; Mittermeier, R.A., Reuter, K.E., Rylands, A.B., Jerusalinsky, L., Schwitzer, C., Strier, K.B., Ratsimbazafy, J., Humle, T., Eds.; UCN SSC Primate Specialist Group, International Primatological Society, Re:wild: Washington, DC, USA, 2022; pp. 135–140. [Google Scholar]
- Spaan, D.; Ramos-Fernández, G.; Schaffner, C.M.; Pinacho-Guendulain, B.; Aureli, F. How survey design affects monkey counts: A case study on individually recognized spider monkeys (Ateles geoffroyi). Folia Primatol. 2018, 88, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Spaan, D.; Burke, C.; McAree, O.; Aureli, F.; Rangel-Rivera, C.E.; Hutschenreiter, A.; Longmore, S.N.; McWhirter, P.R.; Wich, S.A. Thermal infrared imaging from drones offers a major advance for spider monkey surveys. Drones 2019, 3, 34. [Google Scholar] [CrossRef]
- Kays, R.; Sheppard, J.; Mclean, K.; Welch, C.; Paunescu, C.; Wang, V.; Kravit, G.; Crofoot, M. Hot monkey, cold reality: Surveying rainforest canopy mammals using drone-mounted thermal infrared sensors. Int. J. Remote Sens. 2019, 40, 407–419. [Google Scholar] [CrossRef]
- Busia, L.; Smith-Aguilar, S.E.; Aureli, F.; Schaffner, C.M.; Ramos-Fernández, G. Predation attacks on wild spider monkeys (Ateles geoffroyi). Folia Primatol. 2018, 89, 341–346. [Google Scholar] [CrossRef]
- Matsuda, I.; Izawa, K. Predation of wild spider monkeys at La Macarena, Colombia. Primates 2008, 49, 65–68. [Google Scholar] [CrossRef]
- Mourthé, Í. Reactions of white-bellied spider monkeys to a predation attempt by a cougar. Neotrop. Primates 2011, 18, 28–29. [Google Scholar] [CrossRef]
- Dell’Anna, F.; Schino, G.; Aureli, F. Anxiety in Geoffroy’s spider monkeys (Ateles geoffroyi): Can scratching be used as an indicator? Am. J. Primatol. 2022, 84, e23373. [Google Scholar] [CrossRef]
- Maestripieri, D.; Schino, G.; Aureli, F.; Troisi, A. A modest proposal: Displacement activities as an indicator of emotions in primates. Anim. Behav. 1992, 44, 967–979. [Google Scholar] [CrossRef]
- Schino, G.; Perretta, G.; Taglioni, A.M.; Monaco, V.; Troisi, A. Primate displacement activities as an ethopharmacological model of anxiety. Anxiety 1996, 2, 186–191. [Google Scholar] [CrossRef]
- Briseno-Jaramillo, M.; Ramos-Fernández, G.; Palacios-Romo, T.M.; Sosa-López, J.R.; Lemasson, A. Age and social affinity effects on contact call interactions in free-ranging spider monkeys. Behav. Ecol. Sociobiol. 2018, 72, 192. [Google Scholar] [CrossRef]
- Ramos-Fernandez, G.; Smith Aguilar, S.E.; Schaffner, C.M.; Vick, L.G.; Aureli, F. Site Fidelity in Space Use by Spider Monkeys (Ateles geoffroyi) in the Yucatan Peninsula, Mexico. PLoS ONE 2013, 8, 62813. [Google Scholar] [CrossRef] [PubMed]
- Pinel-Ramos, E.J.; Aureli, F.; Wich, S.; Longmore, S.; Spaan, D. Evaluating Thermal Infrared Drone Flight Parameters on Spider Monkey Detection in Tropical Forests. Sensors 2024, 24, 5659. [Google Scholar] [CrossRef]
- Altmann, J. Observational study of behavior: Sampling methods. Behaviour 1974, 49, 227–267. [Google Scholar] [CrossRef]
- Shimooka, Y.; Campbell, C.J.; Di Fiore, A.; Felton, A.M.; Izawa, K.; Link, A.; Nishimura, A.; Ramos-Fernández, G.; Wallace, R.B. Demography and group composition of Ateles. In Spider Monkeys; Cambridge University Press: Cambridge, UK, 2010; pp. 329–348. [Google Scholar]
- Busia, L.; Schaffner, C.M.; Aureli, F. Relationship quality affects fission decisions in wild spider monkeys (Ateles geoffroyi). Ethology 2017, 123, 405–411. [Google Scholar] [CrossRef]
- Schaffner, C.M.; Rebecchini, L.; Ramos-Fernandez, G.; Vick, L.G.; Aureli, F. Spider monkeys (Ateles geoffroyi yucatenensis) cope with the negative consequences of hurricanes through changes in diet, activity budget, and fission–fusion dynamics. Int. J. Primatol. 2012, 33, 922–936. [Google Scholar] [CrossRef]
- Slater, K.Y.; Schaffner, C.M.; Aureli, F. Sex differences in the social behavior of wild spider monkeys (Ateles geoffroyi yucatanensis). Am. J. Primatol. 2009, 71, 21–29. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. 2024. Available online: https://www.R-project.org/ (accessed on 20 January 2024).
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Linear Mixed-Effects Models using Eigen and S4, version 1.1-10, Package Lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Millar, R.B.; Anderson, M.J. Remedies for pseudoreplication. Fish. Res. 2004, 70, 397–407. [Google Scholar] [CrossRef]
- Venables, W.N.; Ripley, B.D. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
- Harrison, X.A. Using observation-level random effects to model overdispersion in count data in ecology and evolution. PeerJ. 2014, 2, e616. [Google Scholar] [CrossRef]
- Shishkina, T.; Farmus, L.; Cribbie, R.A. Testing for a lack of relationship among categorical variables. Quant. Method. Psychol. 2018, 14, 167–179. [Google Scholar] [CrossRef]
- Meyer, D.; Zeileis, A.; Hornik, K.; Gerber, F.; Friendly, M.; Meyer, M.D. Visualizing Categorical Data, version. 1.4-12, Package’vcd.’ CRAN. 2020. Available online: https://cran.r-project.org/web/packages/vcd/index.html (accessed on 1 May 2024).
- Akoglu, H. User’s guide to correlation coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Hijmans, R.J.; Williams, E.; Vennes, C.; Hijmans, M.R.J. Geosphere: Spherical Trigonometry. Version 1.5-18. 2022. Comprehensive R Archive Network (CRAN). Available online: https://CRAN.R-project.org/package=geosphere (accessed on 1 May 2024).
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. Version 0.4.6. 2022. Comprehensive R Archive Network (CRAN). Available online: https://CRAN.R-project.org/package=DHARMa (accessed on 1 June 2024).
- Forstmeier, W.; Schielzeth, H. Cryptic multiple hypotheses testing in linear models: Overestimated effect sizes and the winner’s curse. Behav. Ecol. Sociobiol. 2011, 65, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous Inference in General Parametric Models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef]
- Zeileis, A.; Kleiber, C.; Jackman, S. Regression models for count data in R. J. Stat. Softw. 2008, 27, 1–25. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference. Version 1.47.5. 2023. Comprehensive R Archive Network (CRAN). Available online: https://CRAN.R-project.org/package=MuMIn (accessed on 1 June 2024).
- Frid, A.; Dill, L. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 2002, 6, 11. [Google Scholar] [CrossRef]
- Van Hooff, J.A.R.A.M.; Lukkenaar, B. Captive chimpanzee takes down a drone: Tool use toward a flying object. Primates 2015, 56, 289–292. [Google Scholar] [CrossRef][Green Version]
- Weimerskirch, H.; Prudor, A.; Schull, Q. Flights of drones over sub-Antarctic seabirds show species- and status-specific behavioural and physiological responses. Polar Biol. 2018, 41, 259–266. [Google Scholar] [CrossRef]
- Zumpe, D.; Michael, R.P. Agonistic Behavior. In Notes on the Elements of Behavioral Science; Springer: Boston, MA, USA, 2001; pp. 199–219. [Google Scholar]
- Ortiz-Jiménez, L.; Iglesias-Merchan, C.; Barja, I. Behavioral responses of the European mink in the face of different threats: Conspecific competitors, predators, and anthropic disturbances. Sci. Rep. 2021, 11, 8266. [Google Scholar] [CrossRef]
- Lenzi, J.; Felege, C.J.; Newman, R.; McCann, B.; Ellis-Felege, S.N. Feral horses and bison at Theodore Roosevelt National Park (North Dakota, United States) exhibit shifts in behaviors during drone flights. Drones 2022, 6, 136. [Google Scholar] [CrossRef]
- Geldart, E.A.; Barnas, A.F.; Semeniuk, C.A.D.; Gilchrist, H.G.; Harris, C.M.; Love, O.P. A colonial-nesting seabird shows no heart-rate response to drone-based population surveys. Sci. Rep. 2022, 12, 18804. [Google Scholar] [CrossRef] [PubMed]
- Rush, G.P.; Clarke, L.E.; Stone, M.; Wood, M.J. Can drones count gulls? Minimal disturbance and semiautomated image processing with an unmanned aerial vehicle for colony-nesting seabirds. Ecol. Evol. 2018, 8, 12322–12334. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.J.; Hinke, J.T.; Goebel, M.E.; Perryman, W.L. Drones Minimize Antarctic Predator Responses Relative to Ground Survey Methods: An Appeal for Context in Policy Advice. Front. Mar. Sci. 2021, 8, 648772. [Google Scholar] [CrossRef]
- Wallace, P.; Martin, R.; White, I. Keeping pace with technology: Drones, disturbance and policy deficiency. J. Environ. Plan. Manag. 2018, 61, 1271–1288. [Google Scholar] [CrossRef]

| Type | Behavior | Description |
|---|---|---|
| State | Resting | Lying down, sitting, or dangling in the same place |
| State | Feeding | Manipulating or ingesting plant parts (fruits, leaves, flowers) or some other element of the environment (e.g., water, insects, bark) |
| State | Alarm calling | Emitting alarm vocalizations (they are emitted in long sequences) |
| State | Moving | Any movement beyond 0.5 m from the current position in any direction independent of the drone’s location |
| State | Social | Two individuals perform any of the following interactions: chasing, lunging, grooming, embracing, playing |
| Event * | Whinny | Emitting a whinny vocalization (they are usually emitted discretely, not in sequence) |
| Event * | Agonistic display | Vigorously moving the branches or leaves |
| Event * | Scratching | Self-scratching using the fingers on any own body part |
| Event *# | Vigilance | Being alert, with the head oriented upwards, while paying attention to the surroundings. |
| Event *# | Escape | Rapidly moving from their original location away from the drone’s location (recorded only the first occurrence). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinel-Ramos, E.J.; Aureli, F.; Wich, S.; Petersen, M.F.; Dias, P.A.D.; Spaan, D. The Behavioral Responses of Geoffroy’s Spider Monkeys to Drone Flights. Drones 2024, 8, 500. https://doi.org/10.3390/drones8090500
Pinel-Ramos EJ, Aureli F, Wich S, Petersen MF, Dias PAD, Spaan D. The Behavioral Responses of Geoffroy’s Spider Monkeys to Drone Flights. Drones. 2024; 8(9):500. https://doi.org/10.3390/drones8090500
Chicago/Turabian StylePinel-Ramos, Eduardo José, Filippo Aureli, Serge Wich, Merissa F. Petersen, Pedro A. D. Dias, and Denise Spaan. 2024. "The Behavioral Responses of Geoffroy’s Spider Monkeys to Drone Flights" Drones 8, no. 9: 500. https://doi.org/10.3390/drones8090500
APA StylePinel-Ramos, E. J., Aureli, F., Wich, S., Petersen, M. F., Dias, P. A. D., & Spaan, D. (2024). The Behavioral Responses of Geoffroy’s Spider Monkeys to Drone Flights. Drones, 8(9), 500. https://doi.org/10.3390/drones8090500








