Yield Prediction of Four Bean (Phaseolus vulgaris) Cultivars Using Vegetation Indices Based on Multispectral Images from UAV in an Arid Zone of Peru
Abstract
1. Introduction
2. Materials and Methods
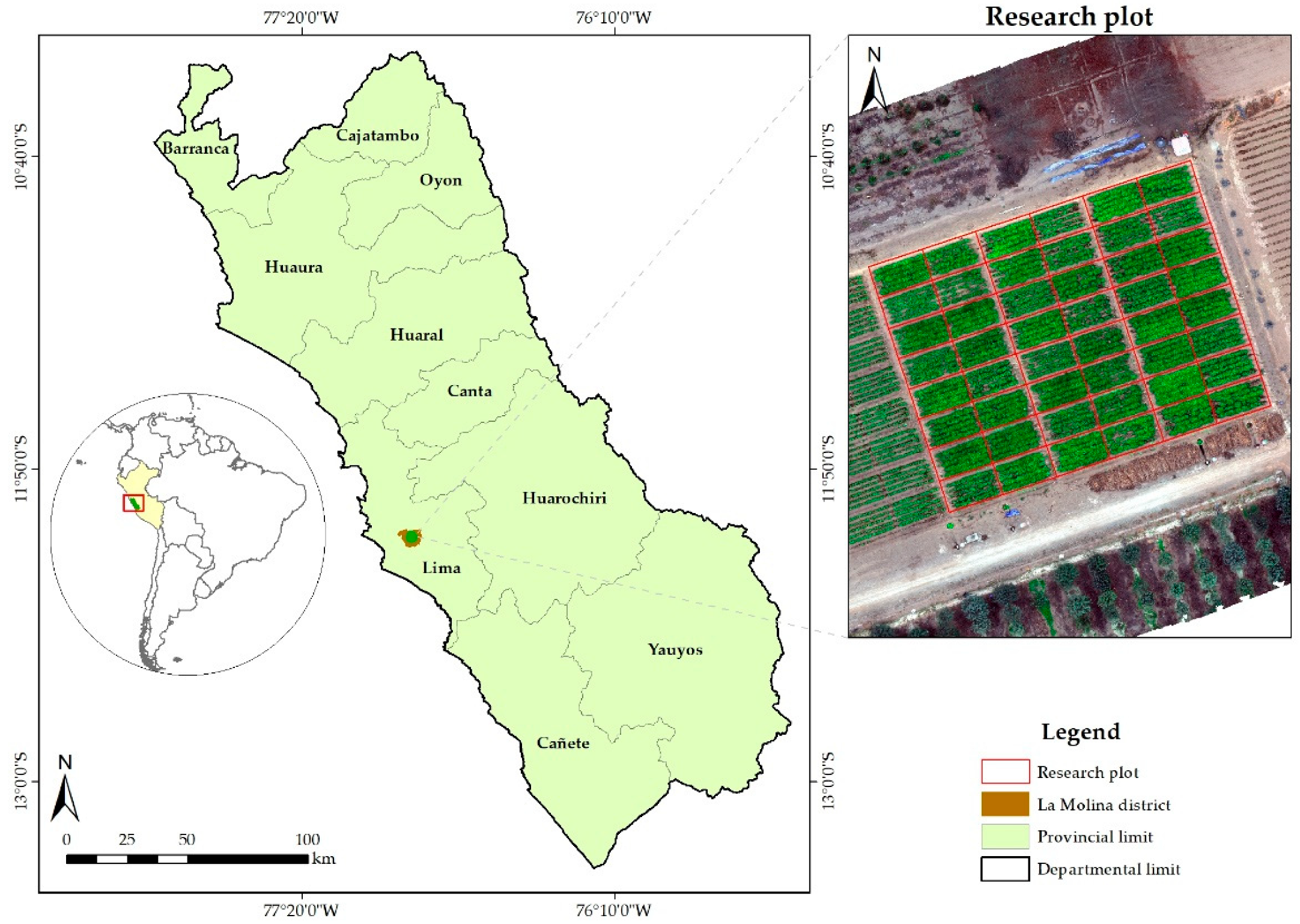
2.1. Study Area
2.2. Plant Height Measurement
2.3. Measurement of Chlorophyll Content
2.4. Measurement of Dry Aerial Biomass and Yield
2.5. Estimation of Vegetation Indices
2.6. Data Analysis and Model Development
3. Results
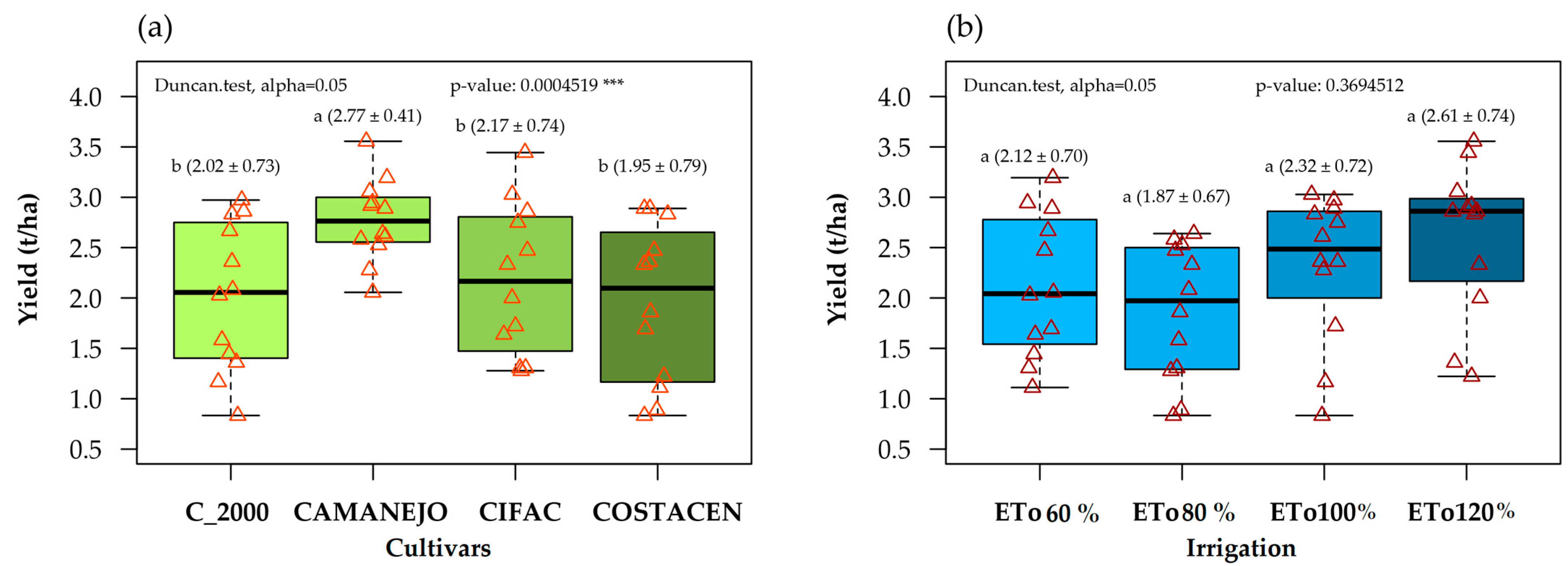
3.1. Effects of Irrigation Treatments and Grain Yield of Bean Varieties
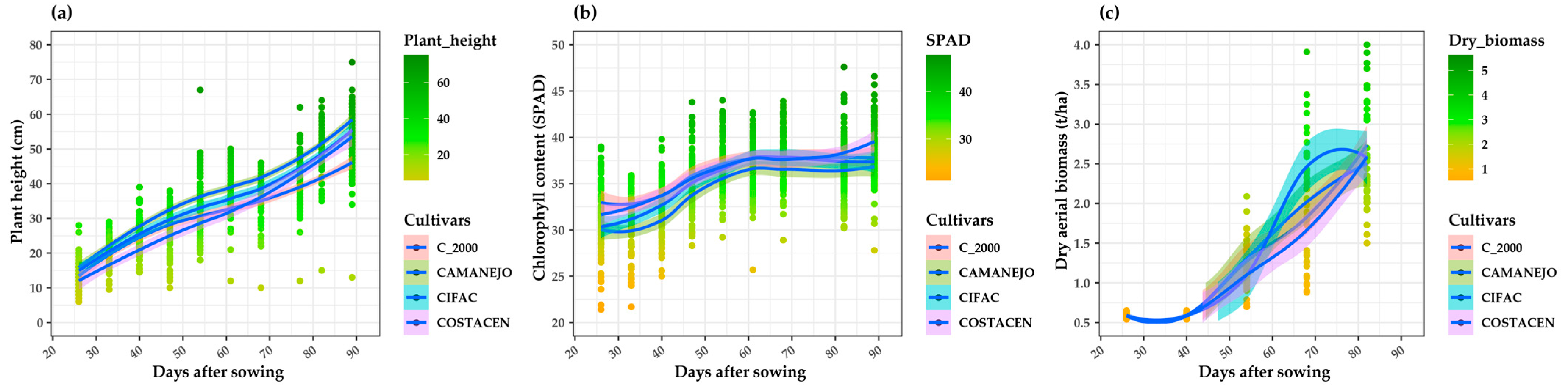
3.2. Variables Evaluated during the Development of the Bean Crop
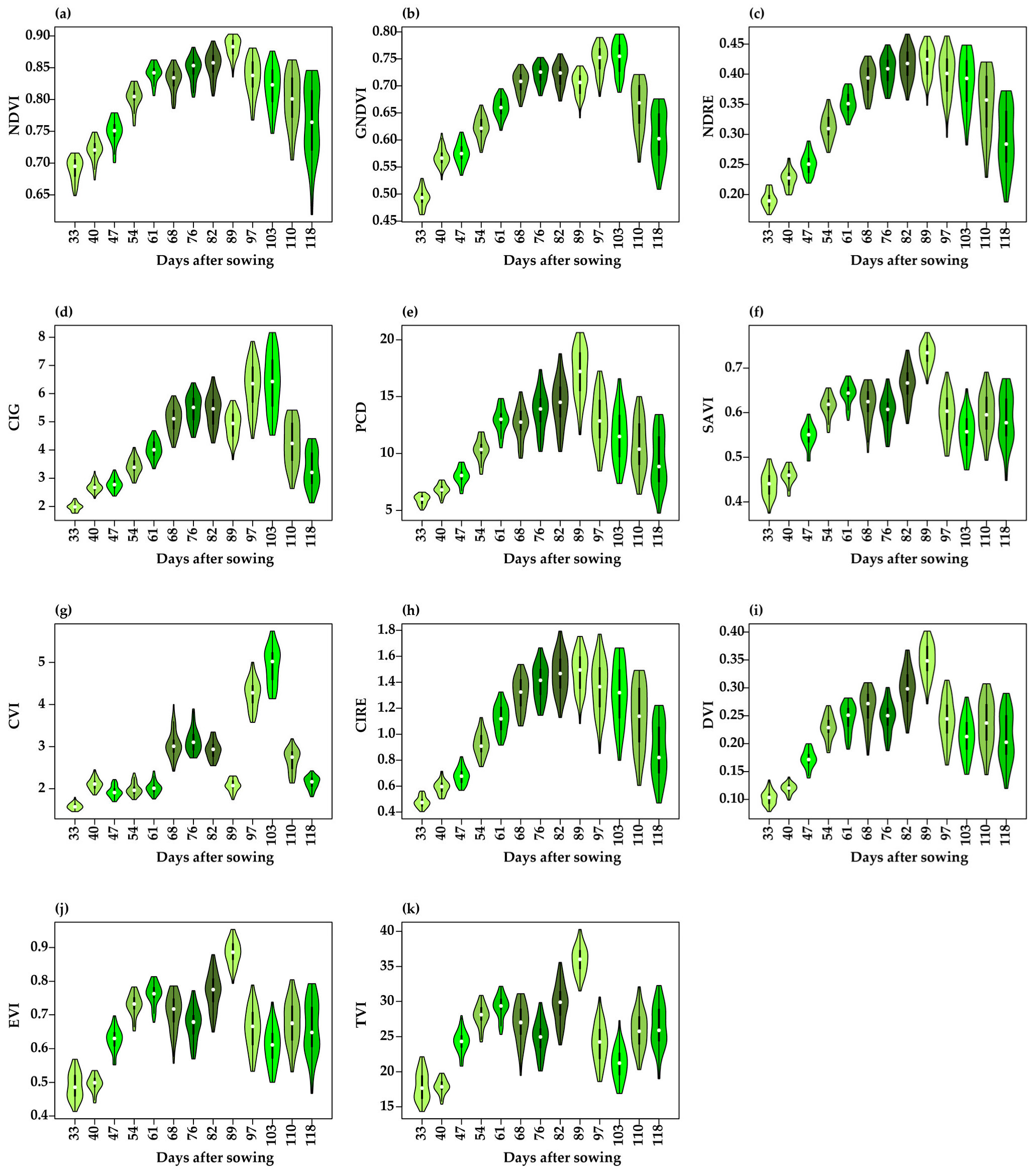
3.3. Vegetation Indices Estimated
3.4. Pearson-r Correlation of Vegetation Indices and Yield
3.5. Multivariate—Principal Components Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Campos-Vega, R.; Reynoso-Camacho, R.; Pedraza-Aboytes, G.; Acosta-Gallegos, J.A.; Guzman-Maldonado, S.H.; Paredes-Lopez, O.; Oomah, B.D.; Loarca-Piña, G. Chemical Composition and in Vitro Polysaccharide Fermentation of Different Beans (Phaseolus vulgaris L.). J. Food Sci. 2009, 74, T59–T65. [Google Scholar] [CrossRef] [PubMed]
- Shamseldin, A.; Velázquez, E. The Promiscuity of Phaseolus vulgaris L. (Common Bean) for Nodulation with Rhizobia: A Review. World J. Microbiol. Biotechnol. 2020, 36, 63. [Google Scholar] [CrossRef] [PubMed]
- Mecha, E.; Figueira, M.; Vaz, M.C.; do Rosário, M. Two Sides of the Same Coin: The Impact of Grain Legumes on Human Health: Common Bean (Phaseolus vulgaris L.) as a Case Study. In Legume Seed Nutraceutical Research; IntechOpen: London, UK, 2019; pp. 26–46. [Google Scholar] [CrossRef]
- MIDAGRI. Plan Nacional de Cultivos: Campaña Agrícola 2019–2020; MIDAGRI: Lima, Perú, 2020; Available online: https://cdn.www.gob.pe/uploads/document/file/471867/Plan_Nacional_de_Cultivos_2019_2020b.pdf (accessed on 13 November 2022).
- Shakoor, N.; Lee, S.; Mockler, T.C. High Throughput Phenotyping to Accelerate Crop Breeding and Monitoring of Diseases in the Field. Curr. Opin. Plant Biol. 2017, 38, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Eggen, M.; Ozdogan, M.; Zaitchik, B.; Ademe, D.; Foltz, J.; Simane, B. Vulnerability of Sorghum Production to Extreme, Sub-Seasonal Weather under Climate Change. Environ. Res. Lett. 2019, 14, 045005. [Google Scholar] [CrossRef]
- Wijesingha, J.; Dayananda, S.; Wachendorf, M.; Astor, T. Comparison of Spaceborne and UAV-Borne Remote Sensing Spectral Data for Estimating Monsoon Crop Vegetation Parameters. Sensors 2021, 21, 2886. [Google Scholar] [CrossRef]
- Puntarenas, P.; Rica, C.; Rica, U.D.C.; Rica, C.; Nacional, U.; Rica, C. Caracterización Espectral y Detección de Flecha Seca En Palma Africana En Puntarenas, Costa Rica. Rev. Geográfica Am. Cent. 2018, 2, 329. [Google Scholar] [CrossRef]
- Gano, B.; Dembele, J.S.B.; Ndour, A.; Luquet, D.; Beurier, G.; Diouf, D.; Audebert, A. Using Uav Borne, Multi-Spectral Imaging for the Field Phenotyping of Shoot Biomass, Leaf Area Index and Height of West African Sorghum Varieties under Two Contrasted Water Conditions. Agronomy 2021, 11, 850. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, H.; Wu, Z.; Wang, S.; Sun, H.; Senthilnath, J.; Wang, J.; Bryant, C.R.; Fu, Y. Modified Red Blue Vegetation Index for Chlorophyll Estimation and Yield Prediction of Maize from Visible Images Captured by Uav. Sensors 2020, 20, 5055. [Google Scholar] [CrossRef]
- Han, L.; Yang, G.; Dai, H.; Xu, B.; Yang, H.; Feng, H.; Li, Z.; Yang, X. Modeling Maize Above-Ground Biomass Based on Machine Learning Approaches Using UAV Remote-Sensing Data. Plant Methods 2019, 15, 1–19. [Google Scholar] [CrossRef]
- Vega, F.A.; Ramírez, F.C.; Saiz, M.P.; Rosúa, F.O. Multi-Temporal Imaging Using an Unmanned Aerial Vehicle for Monitoring a Sunflower Crop. Biosyst. Eng. 2015, 132, 19–27. [Google Scholar] [CrossRef]
- Yue, J.; Feng, H.; Jin, X.; Yuan, H.; Li, Z.; Zhou, C.; Yang, G.; Tian, Q. A Comparison of Crop Parameters Estimation Using Images from UAV-Mounted Snapshot Hyperspectral Sensor and High-Definition Digital Camera. Remote Sens. 2018, 10, 1138. [Google Scholar] [CrossRef]
- Mwinuka, P.R.; Mbilinyi, B.P.; Mbungu, W.B.; Mourice, S.K.; Mahoo, H.F.; Schmitter, P. The Feasibility of Hand-Held Thermal and UAV-Based Multispectral Imaging for Canopy Water Status Assessment and Yield Prediction of Irrigated African Eggplant (Solanum aethopicum L.). Agric. Water Manag. 2021, 245, 106584. [Google Scholar] [CrossRef]
- Parker, T.A.; Palkovic, A.; Gepts, P. Determining the Genetic Control of Common Bean Early-Growth Rate Using Unmanned Aerial Vehicles. Remote Sens. 2020, 12, 1748. [Google Scholar] [CrossRef]
- Santos, A.F.; Lacerda, L.N.; Rossi, C.; Moreno, L.D.A.; Oliveira, M.F.; Pilon, C.; Silva, R.P.; Vellidis, G. Using UAV and Multispectral Images to Estimate Peanut Maturity Variability on Irrigated and Rainfed Fields Applying Linear Models and Artificial Neural Networks. Remote Sens. 2022, 14, 93. [Google Scholar] [CrossRef]
- Saravia, D.; Salazar, W.; Valqui-Valqui, L.; Quille-Mamani, J.; Porras-Jorge, R.; Corredor, F.-A.; Barboza, E.; Vásquez, H.V.; Casas Diaz, A.V.; Arbizu, C.I. Yield Predictions of Four Hybrids of Maize (Zea mays) Using Multispectral Images Obtained from UAV in the Coast of Peru. Agronomy 2022, 12, 2630. [Google Scholar] [CrossRef]
- Quille-Mamani, J.; Porras-Jorge, R.; Saravia-Navarro, D.; Valqui-Valqui, L.; Herrera, J.; Chávez-Galarza, J.; Arbizu, C.I. Assessment of Vegetation Índices Derived from UAV Images for Predicting Biometric Variables in Bean during Ripening Stage. Idesia 2022, 40, 39–45. [Google Scholar] [CrossRef]
- SENAMHI. Mapa Climático del Perú; SENAMHI: Lima, Peru, 2022; Available online: https://www.senamhi.gob.pe/?p=mapa-climatico-del-peru (accessed on 30 April 2022).
- Santos, A.E.; Barbosa, M.R.; de Almeida Moreira, B.R.; da Silva, R.P.; Lemos, L.B. UAV Multispectral Data: A Reliable Approach for Managing Phosphate-Solubilizing Bacteria in Common Bean. Agronomy 2022, 12, 2284. [Google Scholar] [CrossRef]
- Marques, A.P.; Prado, L.; Garcia, D.E.; Nunes, W.; Cordeiro, D.; Ribeiro, L.P.; da Silva, C.A.; Capristo-Silva, G.F.; Li, J.; Rojo, F.E.; et al. A Random Forest Ranking Approach to Predict Yield in Maize with Uav-Based Vegetation Spectral Indices. Comput. Electron. Agric. 2020, 178, 105791. [Google Scholar] [CrossRef]
- Peñuelas, J.; Gamon, J.A.; Griffin, K.L.; Field, C.B. Assessing Community Type, Plant Biomass, Pigment Composition, and Photosynthetic Efficiency of Aquatic Vegetation from Spectral Reflectance. Remote Sens. Environ. 1993, 46, 110–118. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Kaufman, Y.J.; Merzlyak, M.N. Use of a Green Channel in Remote Sensing of Global Vegetation from EOS- MODIS. Remote Sens. Environ. 1996, 58, 289–298. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Quantitative Estimation of Chlorophyll-a Using Reflectance Spectra: Experiments with Autumn Chestnut and Maple Leaves. J. Photochem. Photobiol. B Biol. 1994, 22, 247–252. [Google Scholar] [CrossRef]
- Pearson, R.L.; Miller, L.D. Remote Mapping of Standing Crop Biomass for Estimation of the Productivity of the Shortgrass Prairie. Remote Sens. Environ. 1972, 1, 1355. [Google Scholar]
- Huete, A.R. A soil-adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 25, 295–309. [Google Scholar] [CrossRef]
- Vincini, M.; Frazzi, E.; D’Alessio, P. A Broad-Band Leaf Chlorophyll Vegetation Index at the Canopy Scale. Precis. Agric. 2008, 9, 303–319. [Google Scholar] [CrossRef]
- Gitelson, A.A.; Gritz, Y.; Merzlyak, M.N. Relationships between Leaf Chlorophyll Content and Spectral Reflectance and Algorithms for Non-Destructive Chlorophyll Assessment in Higher Plant Leaves. J. Plant Physiol. 2003, 160, 271–282. [Google Scholar] [CrossRef]
- Duarte, L.; Teodoro, A.C.; Sousa, J.J.; Pádua, L. Qvigourmap: A Gis Open Source Application for the Creation of Canopy Vigour Maps. Agronomy 2021, 11, 952. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.r-project.org/ (accessed on 12 November 2022).
- R Core Team. RStudio: Integrated Development for R. Rstudio Team; PBC: Boston, MA, USA, 2020; Available online: https://www.rstudio.com/authors/rstudio-team/ (accessed on 4 November 2022).
- Schloerke, B.; Cook, D.; Larmarange, J.; Briatte, F.; Marbach, M.; Thoen, E.; Elberg, A.; Crowley, J. Package “GGally”. Available online: https://cran.r-project.org/web/packages/GGally/GGally.pdf (accessed on 12 November 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Cham, Switzerland, 2016. [Google Scholar]
- de Mendiburu, F. Package “Agricolae”. Available online: https://cran.r-project.org/web/packages/agricolae/agricolae.pdf (accessed on 5 November 2022).
- Kassambara, A.; Mundt, F. Package “Factoextra”. Available online: https://cran.r-project.org/web/packages/factoextra/factoextra.pdf (accessed on 12 November 2022).
- Lê, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef]
- Rosales-Serna, R.; Flores-Gallardo, H.; López-González, J.C.; Rubiños-Panta, J.E.; Ortiz-Sánchez, I.A.; Flores-Magdaleno, H.; Santana-Espinoza, S.; Domínguez-Martínez, P.A. Fenología y Productividad Del Agua En Variedades Mejoradas de Frijol Pinto Cultiva-Das En Durango, México. Rev. Fitotec. Mex. 2021, 44, 511. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, A. Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis & Management; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–871. [Google Scholar] [CrossRef]
- Grassini, P.; Thorburn, J.; Burr, C.; Cassman, K.G. High-Yield Irrigated Maize in the Western U.S. Corn Belt: I. On-Farm Yield, Yield Potential, and Impact of Agronomic Practices. Field Crop. Res. 2011, 120, 142–150. [Google Scholar] [CrossRef]
- Sishodia, R.P.; Ray, R.L.; Singh, S.K. Applications of Remote Sensing in Precision Agriculture: A Review. Remote Sens. 2020, 12, 3136. [Google Scholar] [CrossRef]
- Ferrer-Vilca, T.H.; Valverde-Rodríguez, A. Rendimiento del frejol (Phaseolus vulgaris L.) variedad canario con tres fuentes de abonos orgánicos en el distrito de Cholón, Huánuco-Perú. Rev. Investig. Agrar. 2020, 2, 33–44. [Google Scholar] [CrossRef]
- Beebe, S.; Ramirez, J.; Jarvis, A.; Rao, I.M.; Mosquera, G.; Bueno, J.M.; Blair, M.W. Genetic Improvement of Common Beans and the Challenges of Climate Change; John Wiley & Sons: Oxford, UK, 2011; pp. 356–369. [Google Scholar]
- Jeong, C.H.; Park, J.H. Analysis of Growth Characteristics Using Plant Height and NDVI of Four Waxy Corn Varieties Based on UAV Imagery. Korean J. Remote Sens. 2021, 37, 733–745. [Google Scholar] [CrossRef]
- Gebremedhin, A.; Badenhorst, P.; Wang, J.; Giri, K.; Spangenberg, G.; Smith, K. Development and Validation of a Model to Combine NDVI and Plant Height for High-Throughput Phenotyping of Herbage Yield in a Perennial Ryegrass Breeding Program. Remote Sens. 2019, 11, 2494. [Google Scholar] [CrossRef]
- Keles, R.; Bayrak, H.; Imriz, G. Determination of Grain Yield and Leaf Chlorophyll Content of Some Dry Bean (Phaseolus vulgarıs L.) Varieties. J. Glob. Innov. Agric. Soc. Sci. 2019, 7, 53–57. [Google Scholar] [CrossRef]
- Palaniswami, C.; Upadhyay, A.K.; Maheswarappa, H.P. Spectral Mixture Analysis for Subpixel Classification of Coconut. Curr. Sci. 2006, 91, 1706–1711. [Google Scholar]
- Lipovac, A.; Bezdan, A.; Moravčevic, D.M.; Djurovic, N.; Cosic, M.; Benka, P.; Stričevic, R.S. Correlation between Ground Measurements and UAV Sensed Vegetation Indices for Yield Prediction of Common Bean Grown under Different Irrigation Treatments and Sowing Periods. Water 2022, 14, 3786. [Google Scholar] [CrossRef]
- Prudnikova, E.; Savin, I.; Vindeker, G.; Grubina, P.; Shishkonakova, E.; Sharychev, D. Influence of soil background on spectral reflectance of winter wheat crop canopy. Remote Sens. 2019, 11, 1932. [Google Scholar] [CrossRef]
- Ali, A.M.; Abouelghar, M.; Belal, A.A.; Saleh, N.; Yones, M.; Selim, A.I.; Amin, M.E.S.; Elwesemy, A.; Kucher, D.E.; Maginan, S.; et al. Crop Yield Prediction Using Multi Sensors Remote Sensing (Review Article). Egypt. J. Remote Sens. Sp. Sci. 2022, 25, 711–716. [Google Scholar] [CrossRef]
- dela Torre, D.M.G.; Gao, J.; Macinnis-Ng, C. Remote Sensing-Based Estimation of Rice Yields Using Various Models: A Critical Review. Geo-Spat. Inf. Sci. 2021, 24, 580–603. [Google Scholar] [CrossRef]
- Nyéki, A.; Kerepesi, C.; Daróczy, B.; Benczúr, A.; Milics, G.; Nagy, J.; Harsányi, E.; Kovács, A.J.; Neményi, M. Application of Spatio-Temporal Data in Site-Specific Maize Yield Prediction with Machine Learning Methods. Precis. Agric. 2021, 22, 1397–1415. [Google Scholar] [CrossRef]
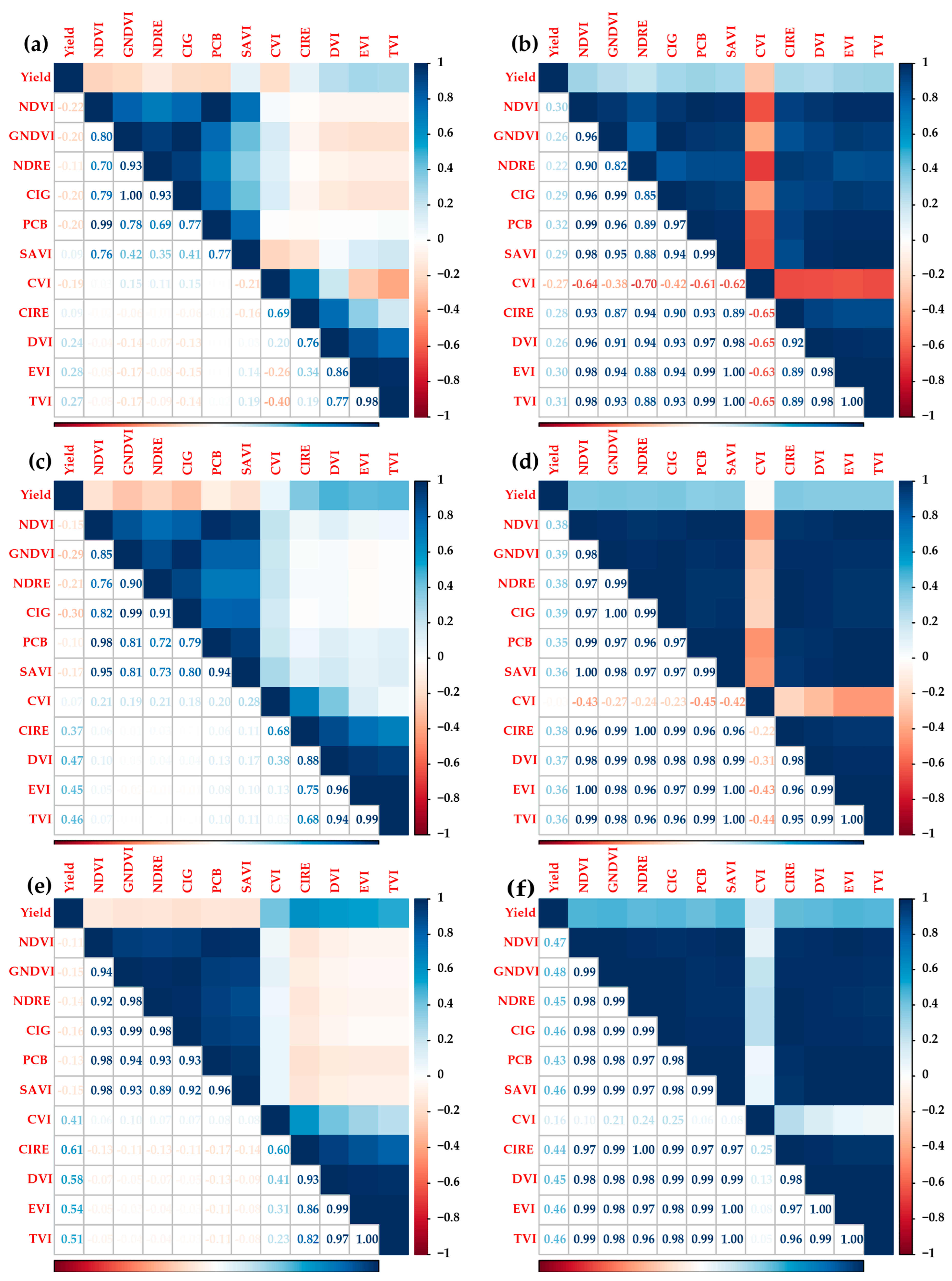
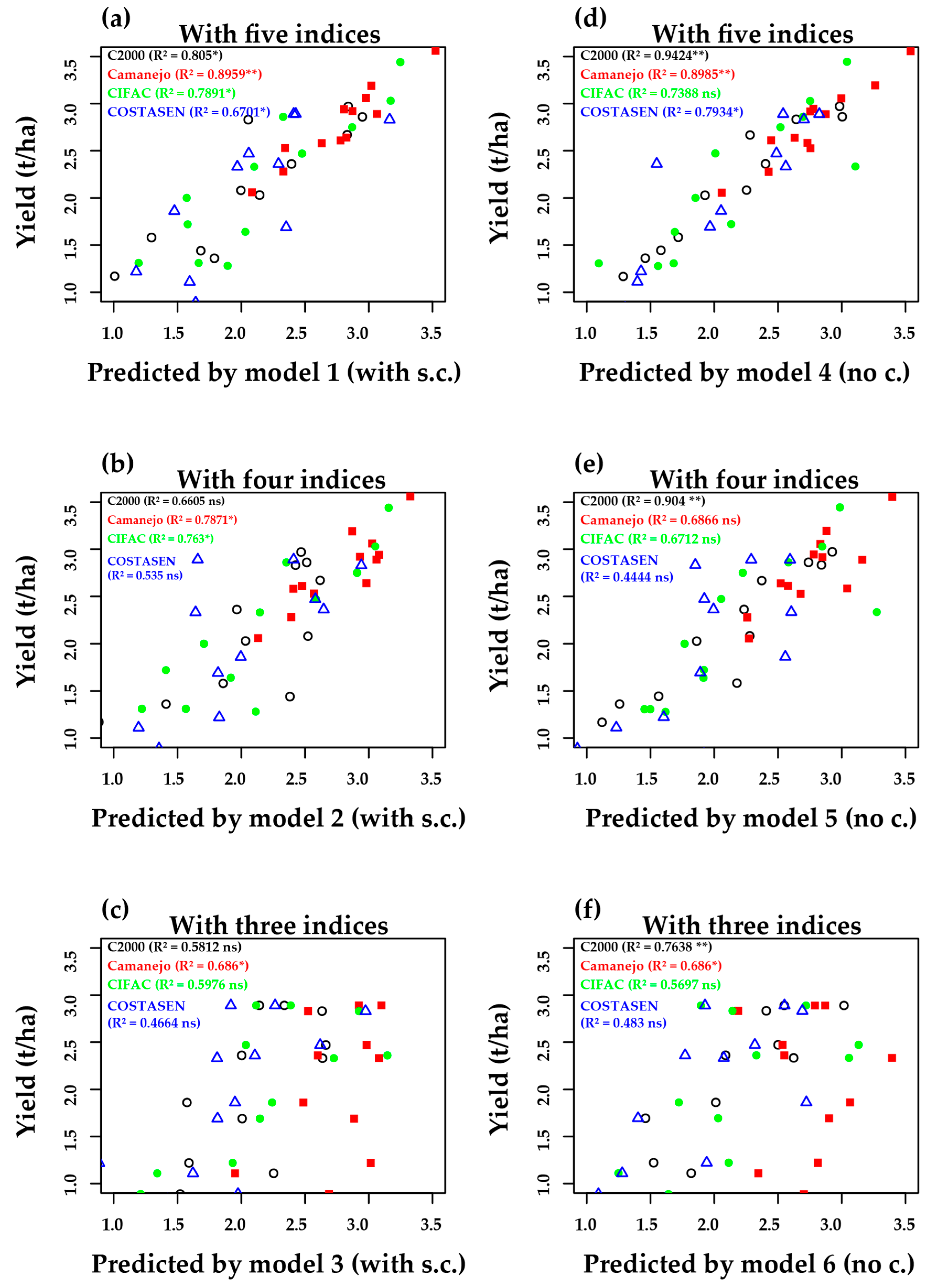
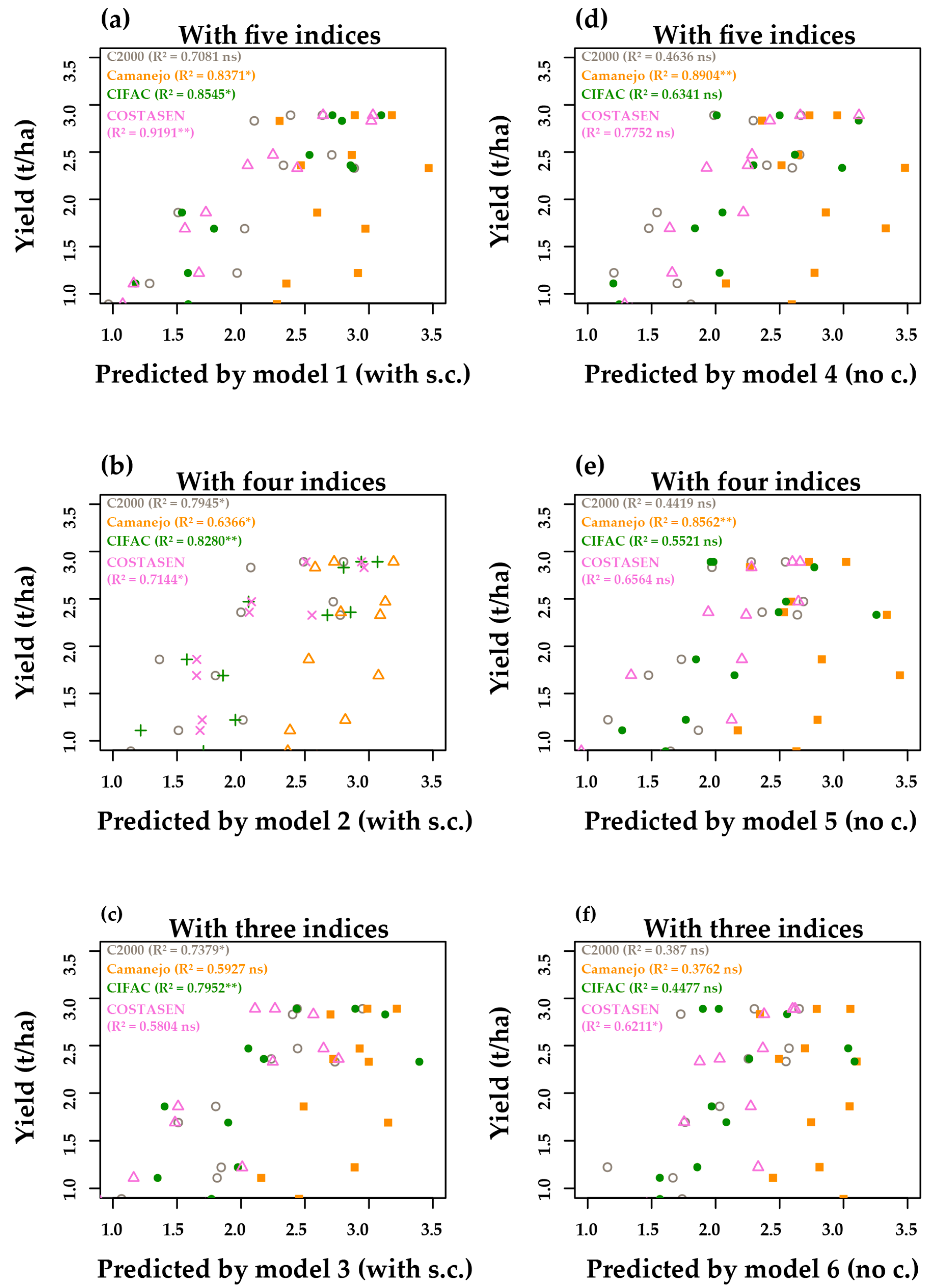


| Indices | Equation | Source |
|---|---|---|
| Normalized Difference Vegetation Index (NDVI) | [22] | |
| Green Normalized Difference Vegetation Index (GNDVI) | [23] | |
| Normalized Difference RedEdge Index (NDRE) | [24] | |
| ChlorophyII Index Green (CIG) | [24] | |
| Plant Cell Density (PCB) | [25] | |
| Soil Adjusted Vegetation Index (SAVI) | [26] | |
| ChlorophyII Vegetation Index (CVI) | [27] | |
| ChlorophyII Index-RedEdge (CIRE) | [28] | |
| Difference Vegetation Index (DVI) | [28] | |
| Enhanced Vegetation Index (EVI) | 2.5 × ((NIR − RED)/((NIR) + (6 × RED) − (7.5 × BLUE) + 1)) | [28] |
| Triangular Vegetation Index (TVI) | 0.5 × (120 × (NIR − GREEN) − 200 × (RED − GREEN) | [28] |
| DAS | NDVI | GNDVI | NDRE | CIG | PCB | SAVI | CVI | CIRE | DVI | EVI | TVI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 33 | −0.259 | −0.158 | −0.127 | −0.159 | −0.298 | * | 0.314 | * | −0.321 | * | −0.23 | 0.001 | 0.138 | 0.130 | ||||||||
| 40 | −0.218 | −0.246 | −0.103 | −0.252 | −0.210 | −0.139 | −0.184 | 0.184 | 0.291 | * | 0.288 | * | 0.281 | |||||||||
| 47 | −0.092 | −0.131 | −0.076 | −0.111 | −0.063 | −0.047 | −0.069 | 0.214 | 0.319 | * | 0.313 | * | 0.317 | * | ||||||||
| 54 | −0.072 | −0.123 | −0.080 | −0.111 | −0.038 | −0.050 | −0.002 | 0.313 | * | 0.360 | * | 0.298 | * | 0.354 | * | |||||||
| 61 | −0.120 | −0.179 | −0.211 | −0.164 | −0.080 | −0.137 | 0.208 | 0.375 | ** | 0.475 | *** | 0.420 | ** | 0.411 | ** | |||||||
| 68 | −0.135 | −0.327 | * | −0.250 | −0.331 | * | −0.079 | −0.168 | 0.035 | 0.399 | ** | 0.496 | *** | 0.500 | *** | 0.489 | *** | |||||
| 76 | −0.008 | 0.013 | 0.016 | 0.008 | −0.039 | −0.024 | 0.184 | 0.534 | *** | 0.514 | *** | 0.453 | *** | 0.429 | *** | |||||||
| 82 | −0.092 | −0.165 | −0.145 | −0.163 | −0.094 | −0.148 | 0.447 | *** | 0.566 | *** | 0.555 | *** | 0.515 | *** | 0.497 | *** | ||||||
| 89 | −0.055 | −0.124 | −0.137 | −0.145 | −0.092 | −0.074 | 0.409 | ** | 0.568 | *** | 0.543 | *** | 0.507 | *** | 0.453 | *** | ||||||
| 97 | −0.109 | −0.116 | −0.097 | −0.131 | −0.103 | −0.125 | 0.417 | ** | 0.628 | *** | 0.620 | *** | 0.599 | *** | 0.576 | *** | ||||||
| 103 | −0.177 | −0.162 | −0.128 | −0.159 | −0.167 | −0.172 | 0.507 | *** | 0.633 | *** | 0.636 | *** | 0.611 | *** | 0.587 | *** | ||||||
| 110 | −0.081 | −0.054 | −0.018 | −0.047 | −0.049 | −0.130 | 0.508 | *** | 0.605 | *** | 0.628 | *** | 0.613 | *** | 0.581 | *** | ||||||
| 118 | −0.452 | ** | −0.389 | ** | −0.377 | ** | −0.394 | ** | −0.453 | ** | −0.460 | ** | 0.046 | 0.515 | *** | 0.559 | *** | 0.575 | *** | 0.591 | *** | |
| DAS | NDVI | GNDVI | NDRE | CIG | PCB | SAVI | CVI | CIRE | DVI | EVI | TVI | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 33 | 0.099 | −0.077 | −0.120 | −0.019 | 0.203 | 0.110 | −0.288 | * | −0.079 | −0.003 | 0.155 | 0.176 | ||||||||||
| 40 | 0.345 | * | 0.316 | * | 0.221 | 0.325 | * | 0.331 | * | 0.313 | * | −0.112 | 0.246 | 0.260 | 0.318 | * | 0.334 | * | ||||
| 47 | 0.363 | * | 0.374 | ** | 0.355 | * | 0.359 | * | 0.342 | * | 0.332 | * | −0.177 | 0.350 | * | 0.321 | * | 0.335 | * | 0.341 | * | |
| 54 | 0.356 | * | 0.345 | * | 0.349 | * | 0.345 | * | 0.345 | * | 0.337 | * | −0.222 | 0.347 | * | 0.332 | * | 0.332 | * | 0.341 | * | |
| 61 | 0.379 | ** | 0.386 | ** | 0.376 | ** | 0.380 | ** | 0.356 | * | 0.356 | * | −0.105 | 0.376 | ** | 0.369 | ** | 0.357 | * | 0.356 | * | |
| 68 | 0.384 | ** | 0.410 | ** | 0.387 | ** | 0.400 | ** | 0.351 | * | 0.381 | ** | 0.086 | 0.386 | ** | 0.380 | ** | 0.382 | ** | 0.377 | ** | |
| 76 | 0.395 | ** | 0.418 | ** | 0.389 | ** | 0.405 | ** | 0.359 | * | 0.384 | ** | 0.195 | 0.384 | ** | 0.378 | ** | 0.382 | ** | 0.378 | ** | |
| 82 | 0.426 | ** | 0.443 | ** | 0.419 | ** | 0.424 | ** | 0.382 | ** | 0.419 | ** | 0.246 | 0.408 | ** | 0.410 | ** | 0.418 | ** | 0.413 | ** | |
| 89 | 0.484 | *** | 0.471 | *** | 0.449 | ** | 0.441 | ** | 0.427 | ** | 0.471 | *** | −0.178 | 0.430 | ** | 0.452 | ** | 0.475 | *** | 0.468 | *** | |
| 97 | 0.540 | *** | 0.526 | *** | 0.487 | *** | 0.494 | *** | 0.494 | *** | 0.536 | *** | 0.175 | 0.464 | *** | 0.504 | *** | 0.533 | *** | 0.529 | *** | |
| 103 | 0.523 | *** | 0.522 | *** | 0.480 | *** | 0.503 | *** | 0.493 | *** | 0.512 | *** | 0.285 | * | 0.466 | *** | 0.483 | *** | 0.508 | *** | 0.501 | *** |
| 110 | 0.533 | *** | 0.507 | *** | 0.474 | *** | 0.482 | *** | 0.491 | *** | 0.540 | *** | 0.108 | 0.457 | ** | 0.505 | *** | 0.544 | *** | 0.543 | *** | |
| 118 | 0.525 | *** | 0.470 | *** | 0.439 | ** | 0.453 | ** | 0.470 | *** | 0.533 | *** | −0.268 | 0.433 | ** | 0.484 | *** | 0.530 | *** | 0.539 | *** | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saravia, D.; Valqui-Valqui, L.; Salazar, W.; Quille-Mamani, J.; Barboza, E.; Porras-Jorge, R.; Injante, P.; Arbizu, C.I. Yield Prediction of Four Bean (Phaseolus vulgaris) Cultivars Using Vegetation Indices Based on Multispectral Images from UAV in an Arid Zone of Peru. Drones 2023, 7, 325. https://doi.org/10.3390/drones7050325
Saravia D, Valqui-Valqui L, Salazar W, Quille-Mamani J, Barboza E, Porras-Jorge R, Injante P, Arbizu CI. Yield Prediction of Four Bean (Phaseolus vulgaris) Cultivars Using Vegetation Indices Based on Multispectral Images from UAV in an Arid Zone of Peru. Drones. 2023; 7(5):325. https://doi.org/10.3390/drones7050325
Chicago/Turabian StyleSaravia, David, Lamberto Valqui-Valqui, Wilian Salazar, Javier Quille-Mamani, Elgar Barboza, Rossana Porras-Jorge, Pedro Injante, and Carlos I. Arbizu. 2023. "Yield Prediction of Four Bean (Phaseolus vulgaris) Cultivars Using Vegetation Indices Based on Multispectral Images from UAV in an Arid Zone of Peru" Drones 7, no. 5: 325. https://doi.org/10.3390/drones7050325
APA StyleSaravia, D., Valqui-Valqui, L., Salazar, W., Quille-Mamani, J., Barboza, E., Porras-Jorge, R., Injante, P., & Arbizu, C. I. (2023). Yield Prediction of Four Bean (Phaseolus vulgaris) Cultivars Using Vegetation Indices Based on Multispectral Images from UAV in an Arid Zone of Peru. Drones, 7(5), 325. https://doi.org/10.3390/drones7050325













