Insecticidal Management of Rangeland Grasshoppers Using a Remotely Piloted Aerial Application System
Abstract
:1. Introduction
2. Materials and Methods
2.1. RPAAS
2.2. Spray Swath and Spray Droplet Spectra Measurements
2.3. Field Trials
3. Results
3.1. Spray Deposition Analysis
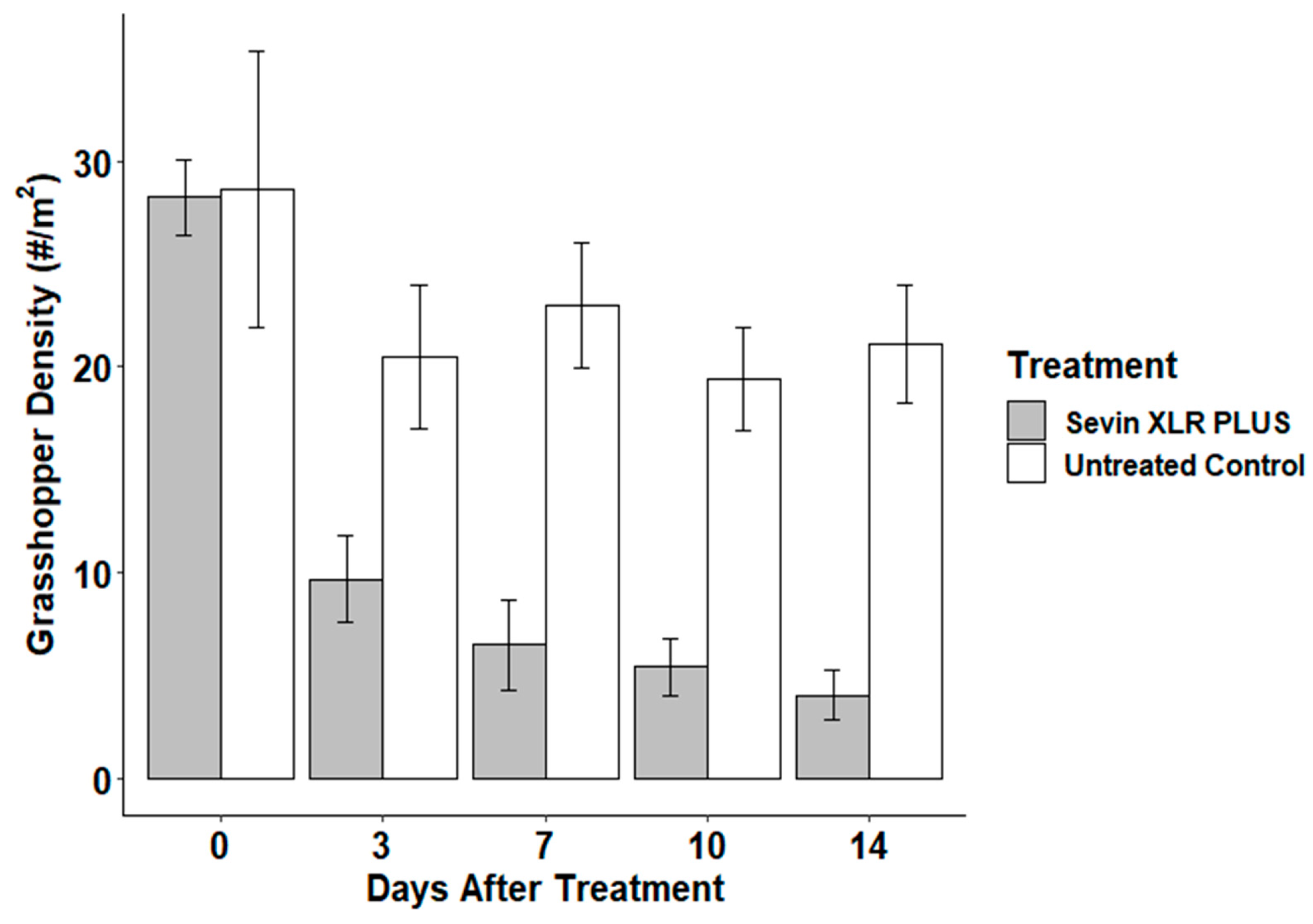
3.2. Field Bioassay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pfadt, R. Field Guide to Common Western Grasshoppers, 3rd ed.; Bulletin No. 912; University of Wyoming, Wyoming Agricultural Experiment Station: Laramie, WY, USA, 2002; pp. 1–288. [Google Scholar]
- Dysart, R.J. VI. 6 Relative importance of rangeland grasshoppers in Western North America: A numerical ranking from the literature. In Grasshopper Integrated Pest Management User Handbook; Cunningham, G., Sampson, M., Eds.; Technical Bulletin No. 1809; USDA Animal and Plant Health Inspection Service: Washington, DC, USA, 2000; pp. 1–20. [Google Scholar]
- Hewitt, G.; Onsager, J. Control of grasshoppers on rangeland in the United States—A perspective. Rangel. Ecol. Manag. J. Range Manag. Arch. 1983, 36, 202–207. [Google Scholar] [CrossRef]
- Belovsky, G.E.; Lockwood, J.A.; Winks, K. IV. 8 Recognizing and managing potential outbreakconditions. In Grasshopper Integrated Pest Management User Handbook; Cunningham, G., Sampson, M., Eds.; Technical Bulletin No. 1809; USDA Animal and Plant Health Inspection Service: Washington, DC, USA, 2000; pp. 1–4. [Google Scholar]
- Branson, D.H.; Joern, A.; Sword, G.A. Sustainable Management of Insect Herbivores in Grassland Ecosystems: New Perspectives in Grasshopper Control. BioScience 2006, 56, 743–755. [Google Scholar] [CrossRef]
- Belovsky, G. Do grasshoppers diminish grassland productivity? A new perspective for control based on conservation. In Grasshoppers and Grassland Health; Springer: Dordrecht, The Netherlands, 2000; pp. 7–29. [Google Scholar]
- Royer, T.A.; Mulder, P. Grasshopper Management in Rangeland, Pastures, and Crops. In Extension Service; Oklahoma State University, Ed.; Oklahoma State University: Stillwater, OK, USA, 2004; Volume EPP-7196, p. 4. [Google Scholar]
- Onsager, J.A. Suppression of grasshoppers in the Great Plains through grazing management. Rangel. Ecol. Manag. J. Range Manag. Arch. 2000, 53, 592–602. [Google Scholar]
- Ball, E.D.; Tinkham, E.; Flock, R.; Vorhies, C. The Grasshoppers and Other Orthoptera of Arizona; College of Agriculture, University of Arizona: Tucson, AZ, USA, 1942; pp. 357–373. [Google Scholar]
- Capinera, J.L.; Thompson, D.C. Dynamics and structure of grasshopper assemblages in shortgrass prairie. Can. Entomol. 1987, 119, 567–575. [Google Scholar] [CrossRef]
- Xiongkui, H.; Bonds, J.; Herbst, A.; Langenakens, J. Recent development of unmanned aerial vehicle for plant protection in East Asia. Int. J. Agric. Biol. Eng. 2017, 10, 18–30. [Google Scholar]
- Shi, Y.; Thomasson, J.A.; Murray, S.C.; Pugh, N.A.; Rooney, W.L.; Shafian, S.; Rajan, N.; Rouze, G.; Morgan, C.L.; Neely, H.L. Unmanned aerial vehicles for high-throughput phenotyping and agronomic research. PLoS ONE 2016, 11, e0159781. [Google Scholar] [CrossRef] [PubMed]
- Giles, D.; Billing, R.; Singh, W. Performance results, economic viability and outlook for remotely piloted aircraft for agricultural spraying. Asp. Appl. Biol. 2016, 132, 15–21. [Google Scholar]
- Yan, X.; Zhou, Y.; Liu, X.; Yang, D.; Yuan, H. Minimizing occupational exposure to pesticide and increasing control efficacy of pests by unmanned aerial vehicle application on cowpea. Appl. Sci. 2021, 11, 9579. [Google Scholar] [CrossRef]
- SAS. SAS Version 9.4; SAS Institute Inc.: Cary, NC, USA, 2012. [Google Scholar]
- Lockwood, J.A.; Schell, S.P.; Foster, R.N.; Reuter, C.; Rachadi, T. Reduced agent-area treatments (RAAT) for management of rangeland grasshoppers: Efficacy and economics under operational conditions. Int. J. Pest Manag. 2000, 46, 29–42. [Google Scholar] [CrossRef]
- ASABE. ASAE Standard S561.1 Procedure for Measuring Drift Deposits from Ground, Orchard, and Aerial Sprayers; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2005. [Google Scholar]
- Berry, J.S.; Onsager, J.A.; Kemp, W.P.; McNary, T.; Larsen, J.; Legg, D.; Lockwood, J.A.; Foster, R.N. VI. 10 Assessing rangeland grasshopper populations. In Grasshopper Integrated Pest Management, User Handbook; Cunningham, G., Sampson, M., Eds.; Technical Bulletin No. 1809; USDA Animal and Plant Health Inspection Service: Washington, DC, USA, 2000; pp. 1–12. [Google Scholar]
- Foster, R.N.; Reuter, K.C. II.2 Evaluation of rangeland grasshopper control: A general protocol for efficacy studies of insecticides applied from the air. In Grasshopper Integrated Pest Management User Handbook; Cunningham, G., Sampson, M., Eds.; Technical Bulletin No. 1809; USDA Animal and Plant Health Inspection Service: Washington, DC, USA, 2000; pp. 1–7. [Google Scholar]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File. Version 5.0/5.0. Available online: http://OrthopteraSpeciesFile.org (accessed on 13 August 2022).
- Connin, R.; Kuitert, L. Control of the American grasshopper with organic insecticides in Florida. J. Econ. Entomol. 1952, 45, 684–687. [Google Scholar] [CrossRef]
- Ku, H.H. Notes on the use of propagation of error formulas. J. Res. Natl. Bureau Standards 1966, 70, 263–273. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]

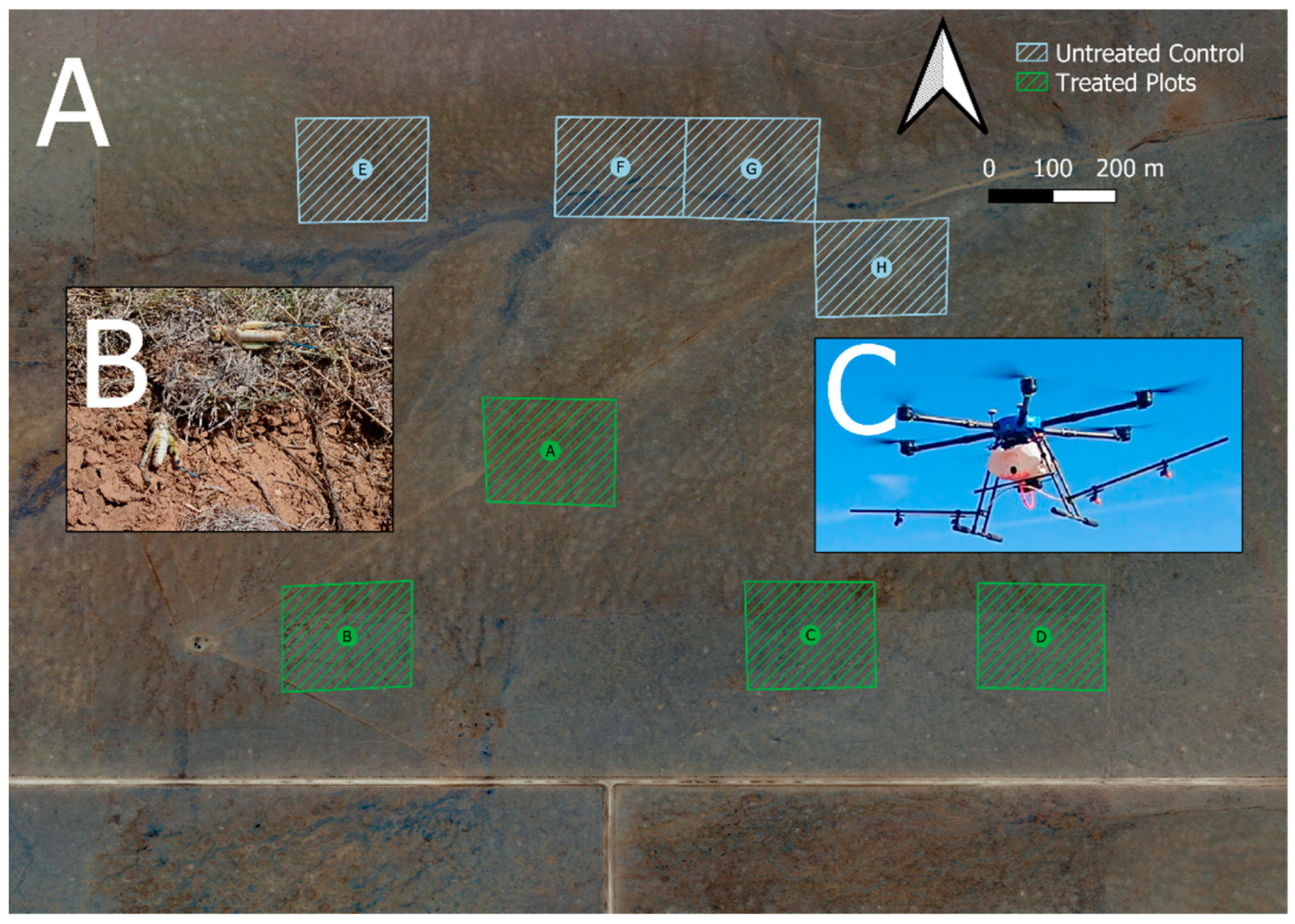

| Pre-Count % Population Composition from Sweeps (100% total per column) | ||||
|---|---|---|---|---|
| Treated Plots | Untreated Control Plots | |||
| Species | Instars 1-5/6 | Adults | Instars 1-5/6 | Adults |
| Acrolophitus hirtipes (Say, 1825) | 0.0% | 0.0% | 0.0% | 0.0% |
| Aeoloplides turnbulli (Thomas, 1872) | 0.0% | 0.0% | 0.0% | 0.0% |
| Ageneotettix deorum (Scudder, 1876) | 9.5% | 0.0% | 6.6% | 0.0% |
| Amphitornus coloradus (Thomas, 1873) | 0.6% | 0.0% | 0.5% | 0.0% |
| Aulocara elliotti (Thomas, 1870) | 23.1% | 31.0% | 24.6% | 59.3% |
| Aulocara femoratum Scudder, 1899 | 12.1% | 0.0% | 24.6% | 0.0% |
| Cordillacris crenulata (Bruner, 1889) | 39.3% | 2.4% | 22.3% | 2.4% |
| Cordillacris occipitalis (Thomas, 1873) | 0.3% | 0.0% | 0.0% | 0.0% |
| Eritettix simplex (Scudder, 1869) | 0.6% | 8.3% | 0.0% | 5.7% |
| Hadrotettix trifasciatus (Say, 1825) | 0.3% | 0.0% | 0.9% | 0.0% |
| Heliaula rufa (Scudder, 1899) | 1.2% | 0.0% | 1.9% | 0.0% |
| Melanoplus occidentalis (Thomas, 1872) | 3.8% | 17.9% | 9.0% | 8.9% |
| Melanoplus packardii Scudder, 1878 | 0.0% | 0.0% | 0.0% | 0.0% |
| Melanoplus regalis (Dodge, 1876) | 0.3% | 0.0% | 0.5% | 0.0% |
| Melanoplus sanguinipes (Fabricius, 1798) | 0.0% | 0.0% | 0.5% | 0.0% |
| Metator pardalinus (Saussure, 1884) | 9.0% | 0.0% | 8.5% | 0.0% |
| Psoloessa delicatula (Scudder, 1876) | 0.0% | 39.9% | 0.0% | 23.6% |
| Xanthippus corallipes (Haldeman, 1852) | 0.0% | 0.6% | 0.0% | 0.0% |
| Pre-Count Overall % Life Stage Presence (100% total) | ||||
| 67.3% | 32.8% | 62.2% | 37.9% | |
| Day 14 Overall % Life Stage Presence (100% Total) | ||||
| 6.3% | 93.8% | 12.2% | 87.8% | |
| Parameter Estimates | Mean ± SEM | Goodness-of-Fit Test (Anderson-Darling) | P > A-Sq | Location: μ0 = 0 t-Statistic |
|---|---|---|---|---|
| Dv0.5 (μm) | 173.5 ± 8.33 | 0.507 | 0.19 | 20.81 * |
| droplet density (#/cm2) | 52.18 ± 4.01 | 0.602 | 0.11 | 13.00 * |
| application rate (L/ha) | 2.75 ± 0.45 | 1.167 | 0.005 * | 6.10 * |
| area coverage (%) | 0.98 ± 0.14 | 1.10 | 0.01 * | 6.77 * |
| Plot | Treatment | Wind Direction | Wind Velocity (m/s) | Temperature (°C) | Relative Humidity (%) |
|---|---|---|---|---|---|
| A | Sevin XLR PLUS | SE | 1.10 | 21.0 | 32.4 |
| B | Sevin XLR PLUS | SE | 2.03 | 21.0 | 34.5 |
| C | Sevin XLR PLUS | SSE | 0.38 | 29.5 | 26.0 |
| D | Sevin XLR PLUS | S | 0.47 | 30.0 | 26.0 |
| Source of Variation | df | Sum of Squares | Mean Square | F | P > F |
|---|---|---|---|---|---|
| treatment | 1 | 660.8 | 660.8 | 13.693 | 0.001 |
| time | 3 | 24.9 | 8.3 | 0.172 | 0.914 |
| treatment: time | 3 | 16.4 | 5.5 | 0.113 | 0.952 |
| residuals | 24 | 1158.1 | 48.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin, D.E.; Rodriguez, R.; Woller, D.A.; Reuter, K.C.; Black, L.R.; Latheef, M.A.; Taylor, M.; López Colón, K.M. Insecticidal Management of Rangeland Grasshoppers Using a Remotely Piloted Aerial Application System. Drones 2022, 6, 239. https://doi.org/10.3390/drones6090239
Martin DE, Rodriguez R, Woller DA, Reuter KC, Black LR, Latheef MA, Taylor M, López Colón KM. Insecticidal Management of Rangeland Grasshoppers Using a Remotely Piloted Aerial Application System. Drones. 2022; 6(9):239. https://doi.org/10.3390/drones6090239
Chicago/Turabian StyleMartin, Daniel E., Roberto Rodriguez, Derek A. Woller, K. Chris Reuter, Lonnie R. Black, Mohamed A. Latheef, Mason Taylor, and Kiara M. López Colón. 2022. "Insecticidal Management of Rangeland Grasshoppers Using a Remotely Piloted Aerial Application System" Drones 6, no. 9: 239. https://doi.org/10.3390/drones6090239
APA StyleMartin, D. E., Rodriguez, R., Woller, D. A., Reuter, K. C., Black, L. R., Latheef, M. A., Taylor, M., & López Colón, K. M. (2022). Insecticidal Management of Rangeland Grasshoppers Using a Remotely Piloted Aerial Application System. Drones, 6(9), 239. https://doi.org/10.3390/drones6090239






