Preliminary Clinical Validation of a Drone-Based Delivery System in Urban Scenarios Using a Smart Capsule for Blood
Abstract
:1. Introduction and Background
The Smart Capsule and the Spoke App
2. Material and Methods
2.1. Validation of the Transport System
2.1.1. Smart Capsule Thermal Stability
2.1.2. Validation of the Integrity of the Flown Blood Products
2.1.3. HFMEA Risk Analysis
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alkouz, B.; Shahzaad, B.; Bouguettaya, A. Service-Based Drone Delivery. In Proceedings of the IEEE 7th International Conference on Collaboration and Internet Computing (CIC), Atlanta, GA, USA, 13–15 December 2021; pp. 68–76. [Google Scholar] [CrossRef]
- Islam, A.; Al Amin, A.; Shin, S.Y. FBI: A Federated Learning-Based Blockchain-Embedded Data Accumulation Scheme Using Drones for Internet of Things. IEEE Wirel. Commun. Lett. 2022, 11, 972–976. [Google Scholar] [CrossRef]
- Hiebert, B.; Nouvet, E.; Jeyabalan, V.; Donelle, L. The Application of Drones in Healthcare and Health-Related Services in North America: A Scoping Review. Drones 2020, 4, 30. [Google Scholar] [CrossRef]
- Cawthorne, D.; Cenci, A. Value Sensitive Design of a Humanitarian Cargo Drone. In Proceedings of the 2019 International Conference on Unmanned Aircraft Systems (ICUAS), 2019, Atlanta, GA, USA, 11–14 June 2019; pp. 1117–1125. [Google Scholar] [CrossRef]
- Rosser, J.C., Jr.; Vignesh, V.; Terwilliger, B.A.; Parker, B.C. Surgical and medical applications of drones: A comprehensive review. J. Soc. Laparoendosc. Surg. 2018, 22, 18. [Google Scholar] [CrossRef] [Green Version]
- Cohen, J. Drone spy plane helps fight California fires. Science 2007, 318, 727. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawabdeh, A.; Moussa, A.; Foroutan, M.; El-Sheimy, N.; Habib, A. Time series UAV image-based point clouds for landslide progression evaluation applications. Sensors 2017, 17, 2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amukele, T. The economics of medical drones. Lancet Glob. Health 2020, 8, e22. [Google Scholar] [CrossRef] [Green Version]
- Cawthorne, D.; Robbins-van Wynsberghe, A. An Ethical Framework for the Design, Development, Implementation, and Assessment of Drones Used in Public Healthcare. Sci. Eng. Ethics 2020, 26, 2867–2891. [Google Scholar] [CrossRef] [PubMed]
- Amukele, T. Current State of Drones in Healthcare: Challenges and Opportunities. J. Appl. Lab. Med. 2019, 4, 296–298. [Google Scholar] [CrossRef]
- Laksham, K.B. Unmanned aerial vehicle (drones) in public health: A SWOT analysis. J. Fam. Med. Prim. Care 2019, 8, 342–346. [Google Scholar] [CrossRef]
- Arcury, T.A.; Preisser, J.S.; Gesler, W.M.; Powers, J.M. Access to transportation and health care utilization in a rural region. J. Rural Health 2005, 2, 31–38. [Google Scholar] [CrossRef]
- Tanser, F.; Gijsbertsen, B.; Herbst, K. Modeling and understanding primary health care accessibility and utilization in rural South Africa: An exploration using a geographical information system. Soc. Sci. Med. 2006, 63, 691–705. [Google Scholar] [CrossRef] [PubMed]
- Nisingizwe, M.P.; Ndishimye, P.; Swaibu, K.; Nshimiyimana, L.; Karame, P.; Dushimiyimana, V.; Musabyimana, J.P.; Musanabaganwa, C.; Nsanzimana, S.; Law, M.R. Effect of unmanned aerial vehicle (drone) delivery on blood product delivery time and wastage in Rwanda: A retrospective, cross-sectional study and time series analysis. Lancet Glob. Health 2022, 10, e564–e569. [Google Scholar] [CrossRef]
- Amukele, T.K.; Sokoll, L.J.; Pepper, D.; Howard, D.P.; Street, J. Can unmanned aerial systems (Drones) be used for the routine transport of chemistry, hematology, and coagulation laboratory specimens? PLoS ONE 2015, 10, e0134020. [Google Scholar] [CrossRef] [Green Version]
- Ling, G.; Draghic, N. Aerial drones for blood delivery. Transfusion 2019, 59, 1608–1611. [Google Scholar] [CrossRef] [Green Version]
- Carrillo-Larco, R.M.; Moscoso-Porras, M.; Taype-Rondan, A.; Ruiz-Alejos, A.; Bernabe-Ortiz, A. The use of unmanned aerial vehicles for health purposes: A systematic review of experimental studies. Glob. Health Epidemiol. Genom. 2018, 3, e13. [Google Scholar] [CrossRef]
- Knoblauch, A.M.; de la Rosa, S.; Sherman, J.; Blauvelt, C.; Matemba, C.; Maxim, L.; Defawe, O.D.; Gueye, A.; Robertson, J.; McKinney, J.; et al. Bi-directional drones to strengthen healthcare provision: Experiences and lessons from Madagascar, Malawi and Senegal. BMJ Glob. Health 2019, 4, e001541. [Google Scholar] [CrossRef] [Green Version]
- Lippi, G.; Mattiuzzi, C. Biological samples transportation by drones: Ready for prime time? Ann. Transl. Med. 2016, 4, 92. [Google Scholar] [CrossRef]
- Amukele, T.K.; Street, J.; Carroll, K.; Miller, H.; Zhang, S.X. Drone transport of microbes in blood and sputum laboratory specimens. J. Clin. Microbiol. 2016, 54, 2622–2625. [Google Scholar] [CrossRef] [Green Version]
- Glauser, W. Blood-delivering drones saving lives in Africa and maybe soon in Canada. Can. Med. Assoc. J. 2018, 190, E88–E89. [Google Scholar] [CrossRef] [Green Version]
- Amukele, T.; Ness, P.N.; Tobian, A.A.R.; Boyd, J.; Street, J. Drone transportation of blood products. Transfusion 2017, 57, 582–588. [Google Scholar] [CrossRef]
- Amicone, D.; Cannas, A.; Marci, A.; Tortora, G. A Smart Capsule Equipped with Artificial Intelligence for Autonomous Delivery of Medical Material through Drones. Appl. Sci. 2021, 11, 7976. [Google Scholar] [CrossRef]
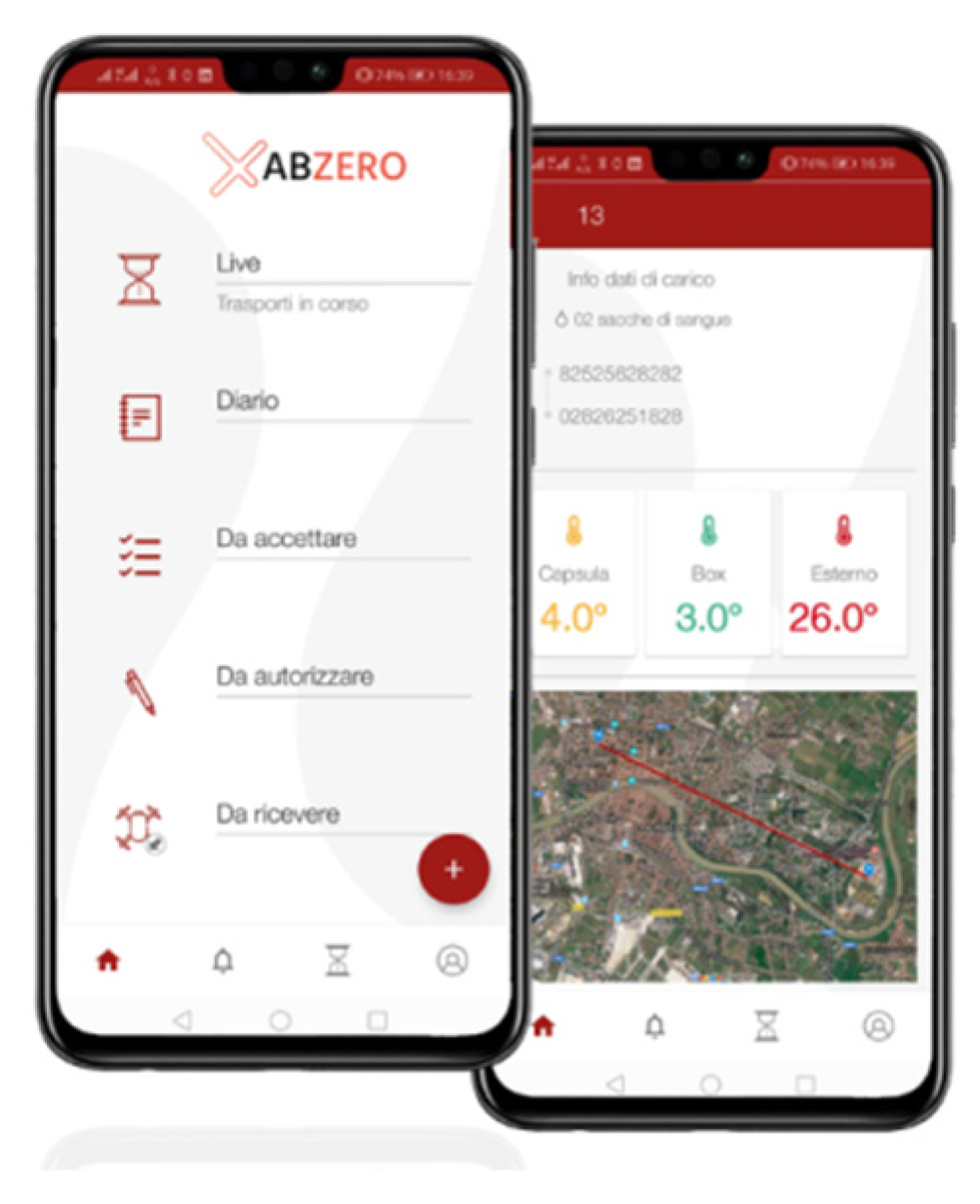




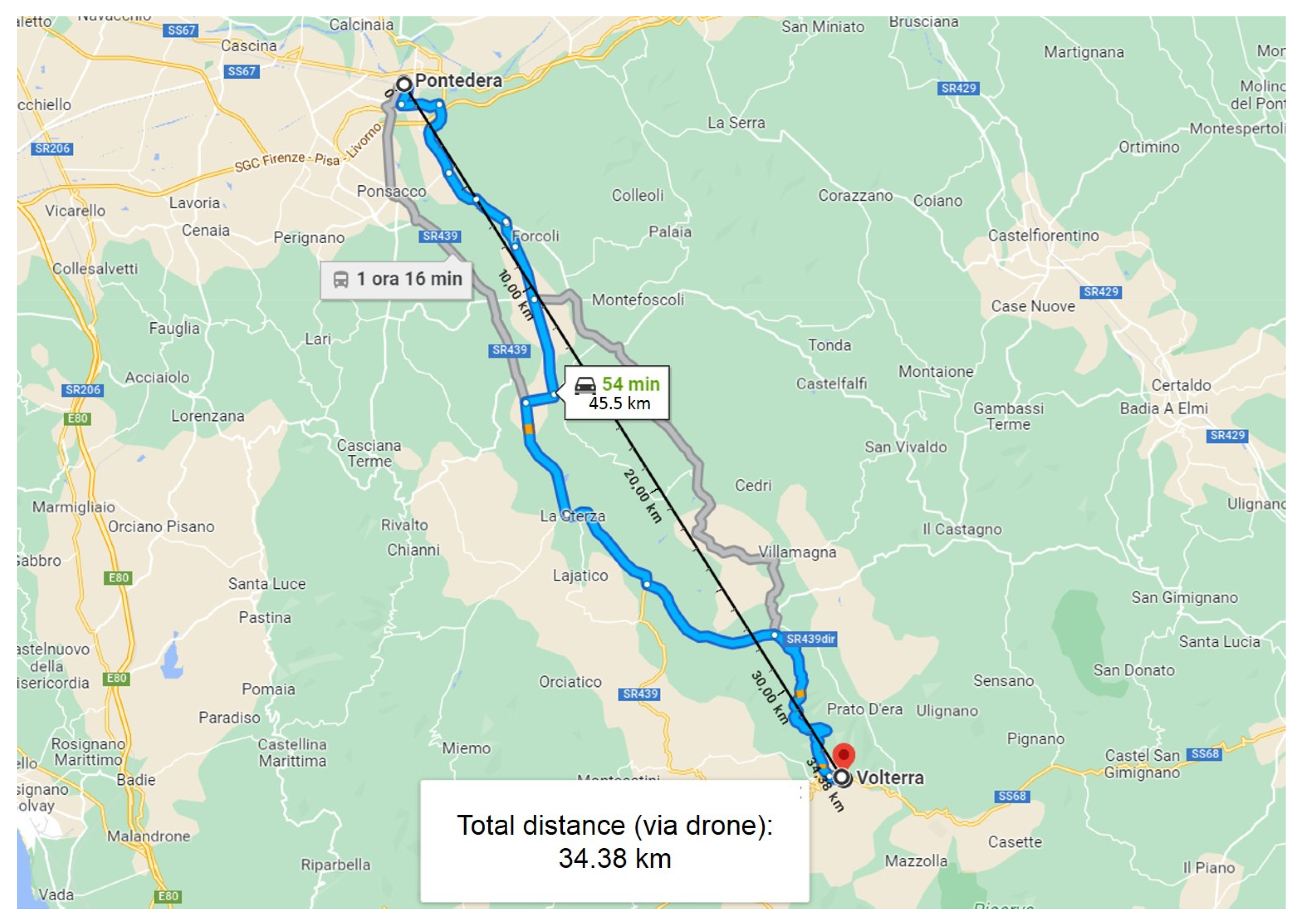
| Day | Duration (min) | Length (m) | Altitude (m) Min/Max | Forecast Weather | Wind (km/h) | Temperature (°C) |
|---|---|---|---|---|---|---|
| 1 | 13 | 6042 | 60/90 | Cloudy | 8 | 6 |
| 2 | 14 | 7594 | 60/90 | Cloudy | 9 | 7 |
| 3 | 10 | 8961 | 60/90 | Sunny | 4 | 11 |
| 4 | 10 | 3802 | 60/150 | Sunny | 5 | 12 |
| 5 | 17 | 7870 | 60/90 | Sunny | 9 | 14 |
| 6 | 16 | 7593 | 60/90 | Sunny | 9 | 13 |
| 7 | 16 | 8961 | 60/90 | Cloudy | 23 | 8 |
| 8 | 15 | 6278 | 60/90 | Cloudy | 25 | 9 |
| Flight | Altitudinal Max (m) | Forecast Weather | Drone Speed Km/h | Wind (km/h) | Temperature (°C) |
|---|---|---|---|---|---|
| 1 | 150 | Cloudy | 60 | No-wind | 6 |
| 2 | 150 | Sunny | 60 | No-wind | 8–9 |
| 3 | 150 | Sunny | 60 | 23 | 22 |
| 4 | 150 | Serene | 60 | 29 | 6.5 |
| Potassium (K) | Value (mEq/L) | Reference Values |
|---|---|---|
| Antes-fligth | 3.79 | 3.60–5.50 |
| Post-fligth | 3.95 | 3.60–5.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niglio, F.; Comite, P.; Cannas, A.; Pirri, A.; Tortora, G. Preliminary Clinical Validation of a Drone-Based Delivery System in Urban Scenarios Using a Smart Capsule for Blood. Drones 2022, 6, 195. https://doi.org/10.3390/drones6080195
Niglio F, Comite P, Cannas A, Pirri A, Tortora G. Preliminary Clinical Validation of a Drone-Based Delivery System in Urban Scenarios Using a Smart Capsule for Blood. Drones. 2022; 6(8):195. https://doi.org/10.3390/drones6080195
Chicago/Turabian StyleNiglio, Fabrizio, Paola Comite, Andrea Cannas, Angela Pirri, and Giuseppe Tortora. 2022. "Preliminary Clinical Validation of a Drone-Based Delivery System in Urban Scenarios Using a Smart Capsule for Blood" Drones 6, no. 8: 195. https://doi.org/10.3390/drones6080195
APA StyleNiglio, F., Comite, P., Cannas, A., Pirri, A., & Tortora, G. (2022). Preliminary Clinical Validation of a Drone-Based Delivery System in Urban Scenarios Using a Smart Capsule for Blood. Drones, 6(8), 195. https://doi.org/10.3390/drones6080195








