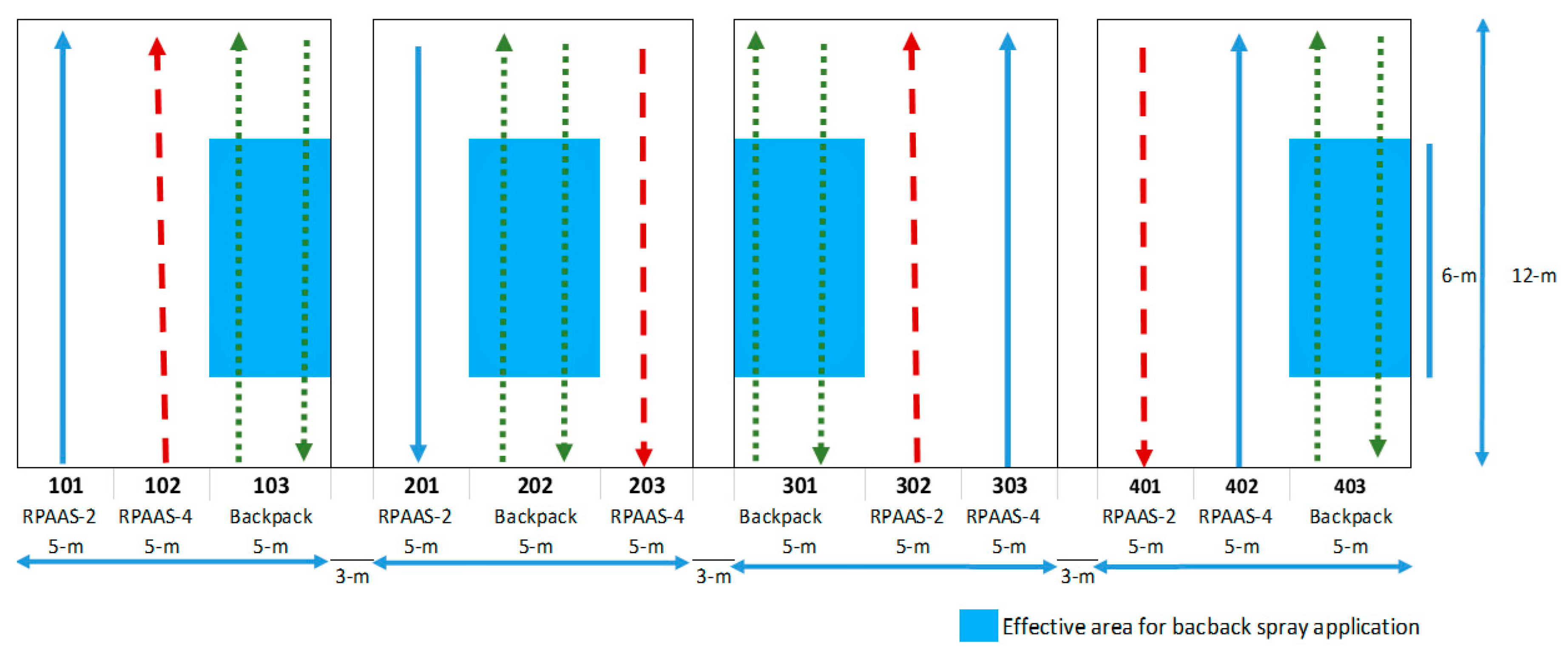
The field experiments were conducted at Texas A&M research farm near College Station, TX, USA (30°32′17″ N; 96°25′19″ W). Four blocks, each 15 m wide × 12 m long, with 3 m between each block, were established with a 5 m strip of land earmarked at random for each treatment (
Figure 1). The experimental units were assembled in a randomized complete block design with four replications to overcome heterogeneity in field conditions, relative to weed density and edaphic conditions between replicated blocks. Soybean was drill seeded at 320,000 seeds·ha
−1 with 76 cm row spacing on 15 May 2018. Weed seeds were broadcasted after soybean planting and were lightly incorporated into the soil. Weed density and size were recorded before spray application. Palmer amaranth and ivyleaf morningglory densities were 19 and 28 plants·m
−2, respectively at the time of spray application. The widest areas of the leaf blades of Palmer amaranth were ca. 7 cm long and 4 cm wide, while those of ivyleaf morning glory were ca. 9 cm long and 7 cm wide when the test was conducted.
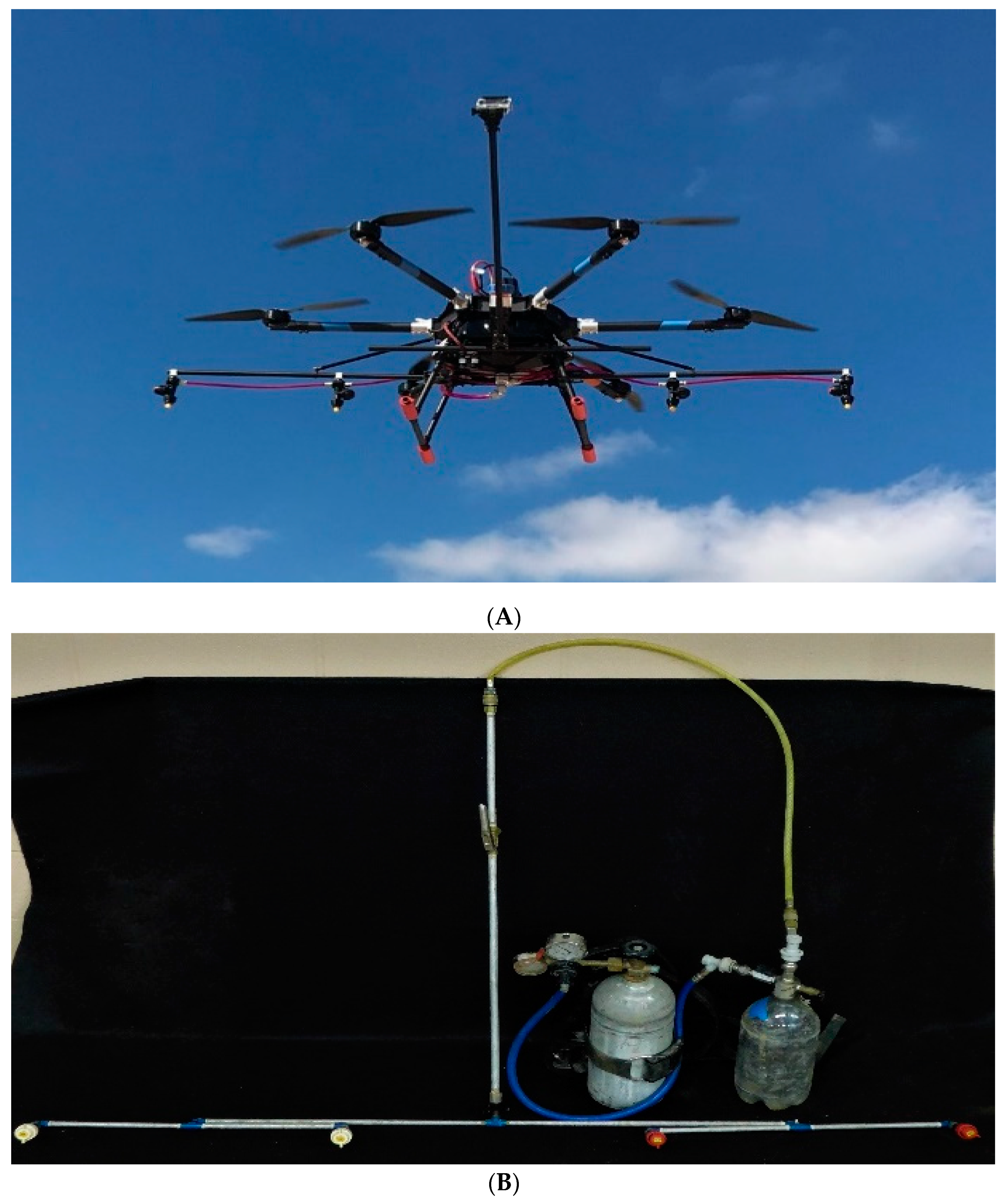
Two spray treatments (18.7 and 37.4 L·ha
−1) were applied with a RPAAS (model V6A, Homeland Surveillance and Electronics, Seattle, WA), and one treatment (140 L·ha
−1) was applied with a custom-made CO
2 backpack sprayer (
Figure 2A,B), respectively, show V6A aircraft and backpack sprayer). These treatments are described in the text for brevity as acronyms, RPAAS-2, RPAAS-4, and BP-15, each representing spray application rates of 2, 4 and 15 gallons per acre or 18.7, 37.4 and 140 L·ha
−1, respectively. This study was conducted on 27 June 2018 (Study 1) and was repeated in time and space two weeks later on 11 July 2018 (Study 2). The details about the spray treatment setups, operating parameters for the backpack sprayer and the RPAAS aircraft, including spray pressure, spray rates, nozzle type and orifice, aircraft ground speed, walking speed for the backpack sprayer and application height are provided in
Table 1. Initially, four TTI110-015 nozzles were installed on the RPAAS. However, due to the low pump capacity, a pressure of only 345 kPa was achievable. For the RPAAS, the outboard nozzles were positioned 0.41 m away from the inboard nozzles, which were 0.82 m apart (
Figure 3). Spray pattern testing was conducted with this setup according to the conventional pattern-testing technique described earlier [
25]. Briefly, four spray passes were conducted with water and fluorescent dye and patterns from each of the passes were averaged to yield a representative spray pattern for the aircraft. The results showed a symmetrical pattern with an effective swath of 4.6 m (15 ft) (
Figure 4). During the field study, only three of the four nozzles were operational (the left outboard nozzle was non-functional), likely due to the daylight visible dye used in the study for image analysis, which reduced system pressure. This pressure was not enough to open the check valves on all the nozzles. As a result, only three of the four nozzles were operational for Study 1, and a full spray pattern for each nozzle was not achieved. With an effective swath of 4.6 m and a ground speed of 4 m·s
−1 for three operational nozzles, the resulting spray application rate was 17.8 L·ha
−1. Two of these passes would yield an application rate of 35.6 L·ha
−1. For Study 2, only the two inboard nozzles were used to achieve a pressure of 414 kPa, which activated both nozzles and provided a full spray pattern for each nozzle. Spray pattern testing for this two-nozzle configuration was not conducted.
The spray solution was comprised of a daylight visible fluorescent dye (Tintex Rocket Red, TX-13, DayGlo Color Corporation, Cleveland, OH, USA) at 10%
v/
v mixed with tap water. The fluorescent dye was used to quantify spray droplets on Palmer amaranth and morningglory leaves using the digital imaging technique documented previously [
32,
33].
2.1. Sampling of Spray Deposition
Water-sensitive paper (WSP) samplers (26 mm × 76 mm) (Spraying Systems, Wheaton, Ill.) were paper clipped to a metal plate (100 mm × 100 mm) and placed on a 0.3 m × 0.3 m wooden board. WSPs were oriented towards the upwind side of the metal plate to keep them flat and secured. There were four artificial samplers placed in each backpack treatment plot and five samplers in each RPAAS treatment plot. WSP samplers were diagonally placed in the test plots with the first sampling location 2 m in from the edge of each plot and subsequent locations 2 m farther down and 1 row over from the previous location.
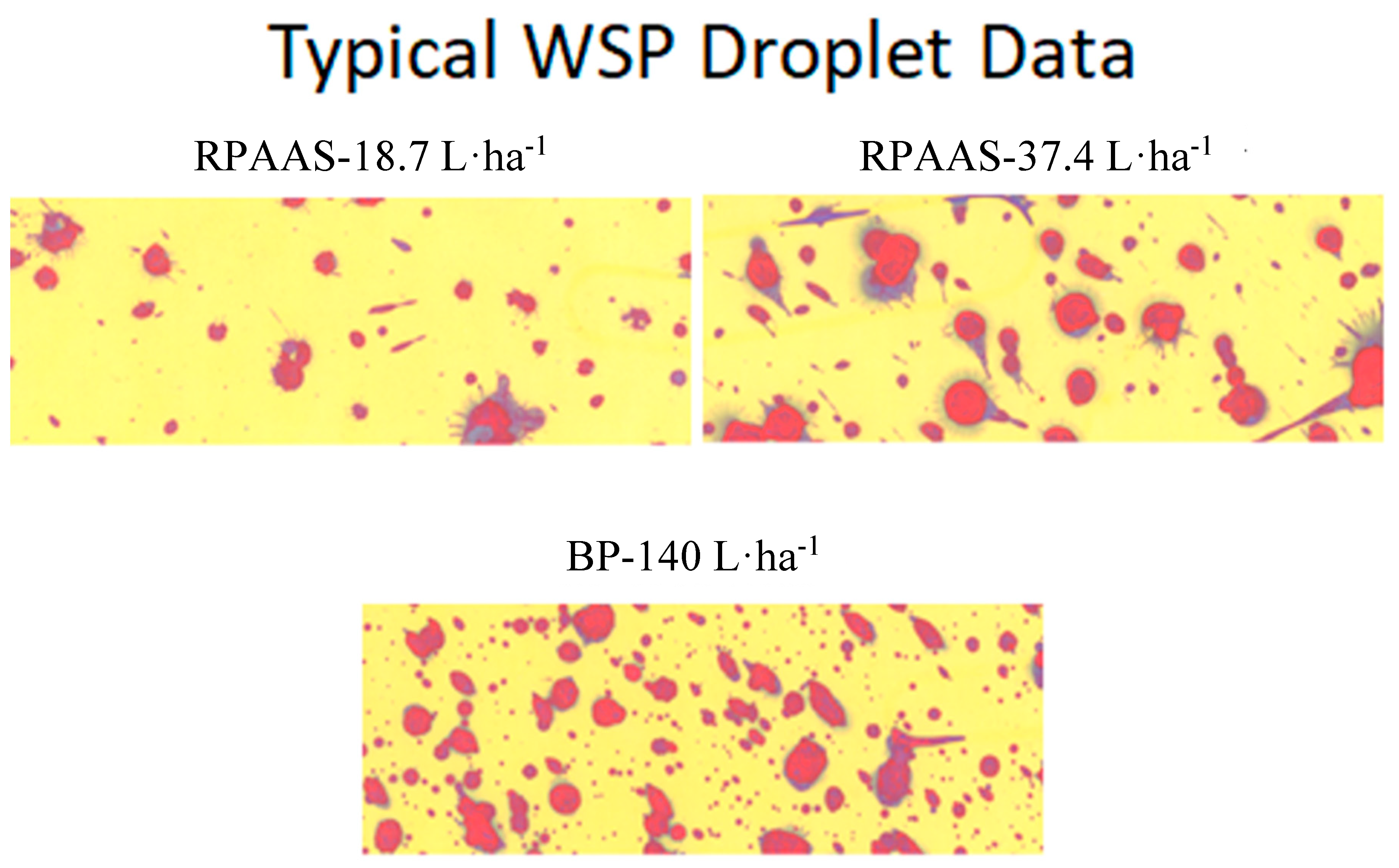
After five minutes of drying time, WSPs were placed inside film negative sleeves. The spray droplets (
Figure 5) captured on them were analyzed in the laboratory by the DropletScan™ scanner-based software system [
34]. The droplet spectra parameters examined were D
v0.1, D
v0.5, D
v0.9, droplet density (droplets/cm
2), percent area coverage and spray efficiency (proportion of spray relative to the target application rate). D
v0.1 is the droplet diameter (µm), where 10% of the spray volume is contained in droplets smaller than this value. Similarly, D
v0.5 and D
v0.9 are droplet diameters, where 50% and 90% of the spray volumes, respectively, are contained in droplets smaller than these values.
2.2. Fluorescent Imaging
Five leaves of Palmer amaranth and morningglory were collected from each replicated block close to where the wooden boards containing the WSP samplers were placed. A total of 20 leaf samples of each weed species were collected from each treatment for the RPAAS platform. However, four leaves of each species were collected for the BP-15 treatment, with a total of 16 leaf samples per species per treatment. Leaves were imaged in the laboratory using a digital single-lens reflex camera (Model Alpha 7R, Sony Corp., Tokyo, Japan), secured to an adjustable camera stand. The camera was equipped with a macro lens (Close-up + 4 Polaroid) and UV filter (49 mm) to help zoom in and obtain a closer view of the droplets, while maintaining high spatial resolution (
ca. 30 µm). Each leaf was placed on an integrated platform at the base of the camera stand where the abaxial and adaxial surfaces of the leaves were imaged. A blue LED light at a wavelength of 470 nm (StellarNet Inc., Tampa, FL, USA) illuminated the droplets during the imaging process. After imaging, the photographs of both the top and bottom leaf surfaces were processed using ImageJ, a public domain, Java-based image processing software. The image processing procedure used in the study was similar to that described earlier by Martin [
32]. However, some modifications were made to accommodate the larger droplet spectrum produced by the TT110-015 nozzles used in the study. Lab color space was used to detect the droplets, with the red threshold color chosen to align with the Rocket Red color of the fluorescent dye. In Lab color space, the ‘L’, ‘a’ and ‘b’ minimum and maximum values were set to 55 and 255, 179 and 205, and 52 and 115, respectively. Spray droplet particles were determined by setting the minimum and maximum pixel area size of the droplets between 10 and 4000 pixels. Circularity values were set between 0.00 and 1.0 to include all of the droplets, regardless of shape. The Show: Outlines option displayed the outlines of the individual droplets and the images were saved for the top and the bottom leaf surfaces.
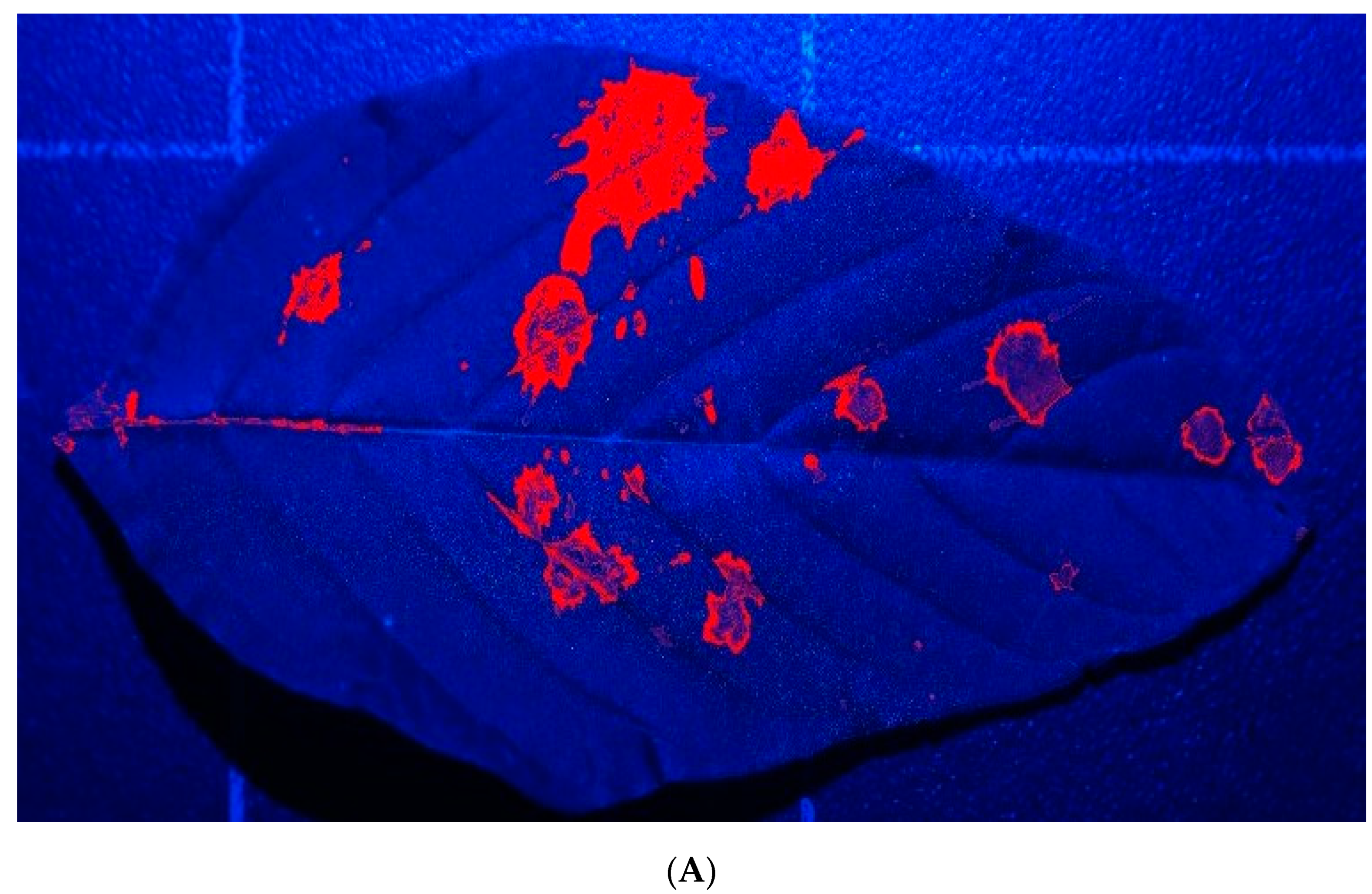
Figure 6 illustrates the enhanced (
Figure 6A), selected (
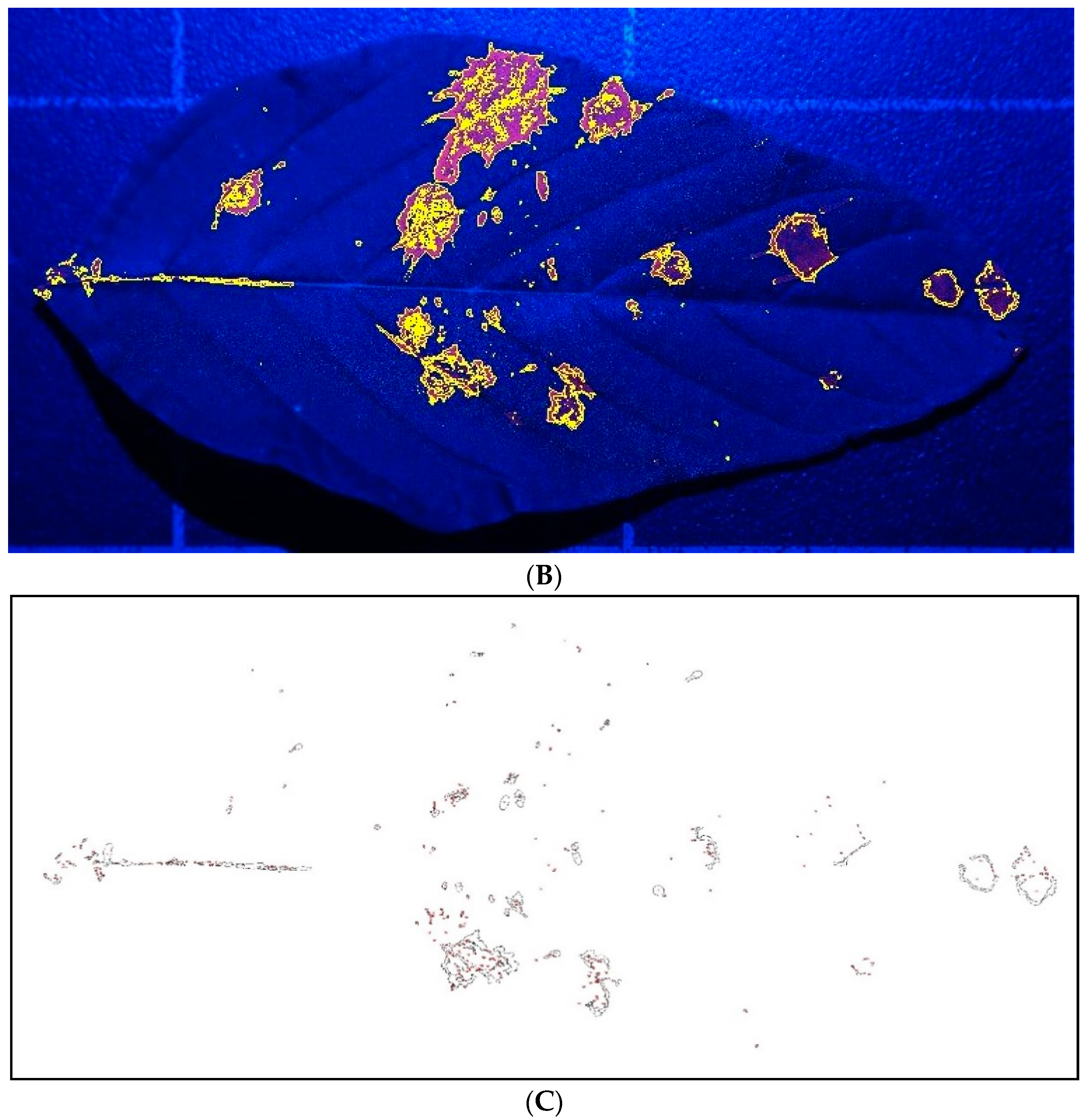
Figure 6B) and computer drawings (
Figure 6C) of the image of spray droplets on the top surface of a Palmer amaranth leaf as an example.
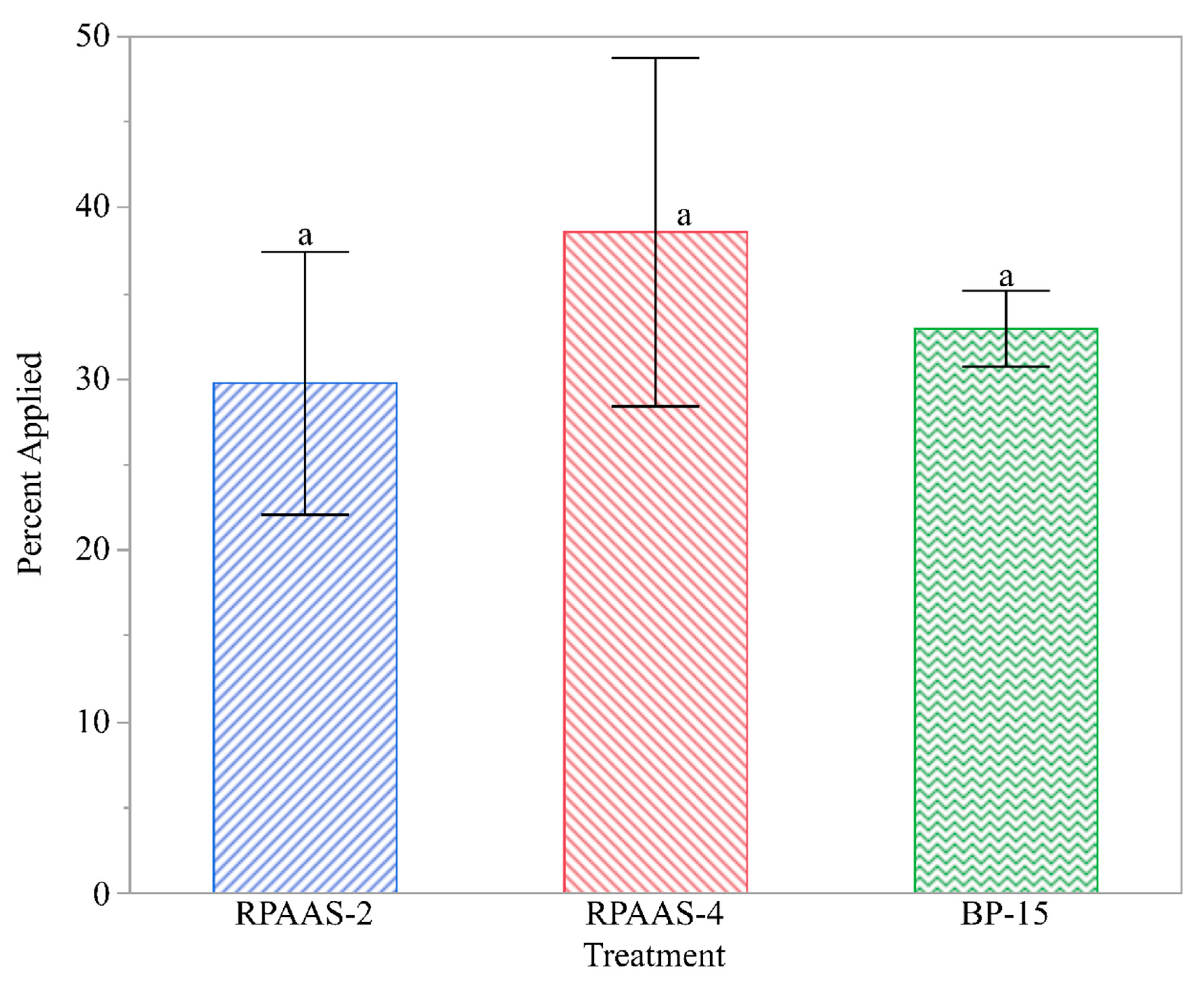
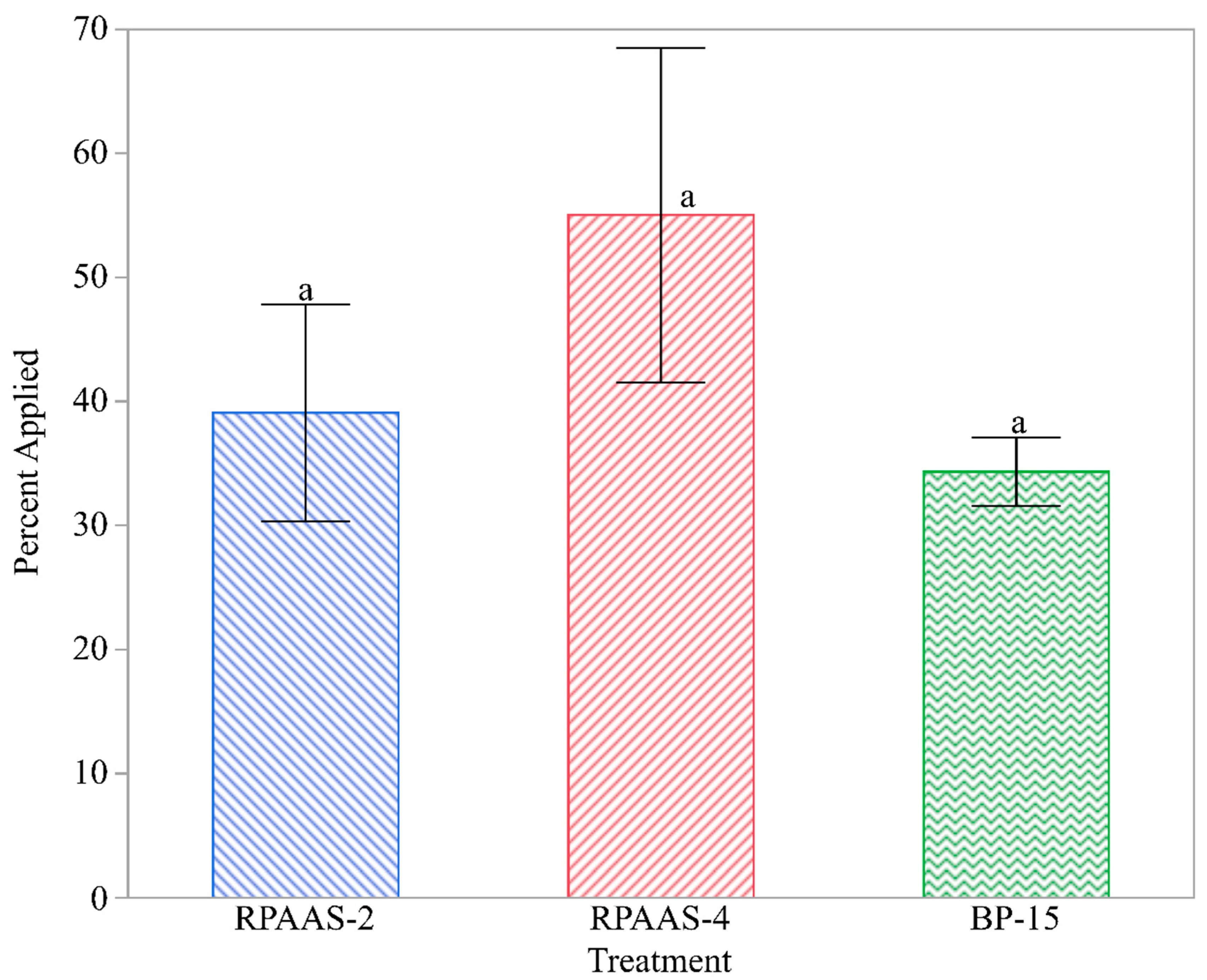
Thus, the fluorescent imaging provided data on the number of droplets found in each sample of leaves collected. Spray droplet density (droplets/cm2) on the adaxial and abaxial surfaces of the weed foliage was calculated using the area of each leaf determined by a leaf area meter (Model 3100, Li-Cor, Lincoln, NE, USA). Because the droplet density data were highly variable, the percentage of spray droplets on the adaxial and abaxial surfaces of the leaves was calculated for each sample collected from the designated locations in each treated block to compare the treatments using a common format.
2.3. Data Analysis
Spray deposits, as a percentage of the target application rate, were highly variable and comprised of values ranging from 0 to 100%. Because of the high variability of the data, they were transformed to arcsine
, where
p = original variates in proportions [
35]. The spray droplet density of the fluorescent imaging data was transformed to log (
x + 1), where
x = the original variate. When the mean is positively correlated with the variance, the logarithmic transformation is likely to remedy the situation, and make the variance independent of the mean [
36].
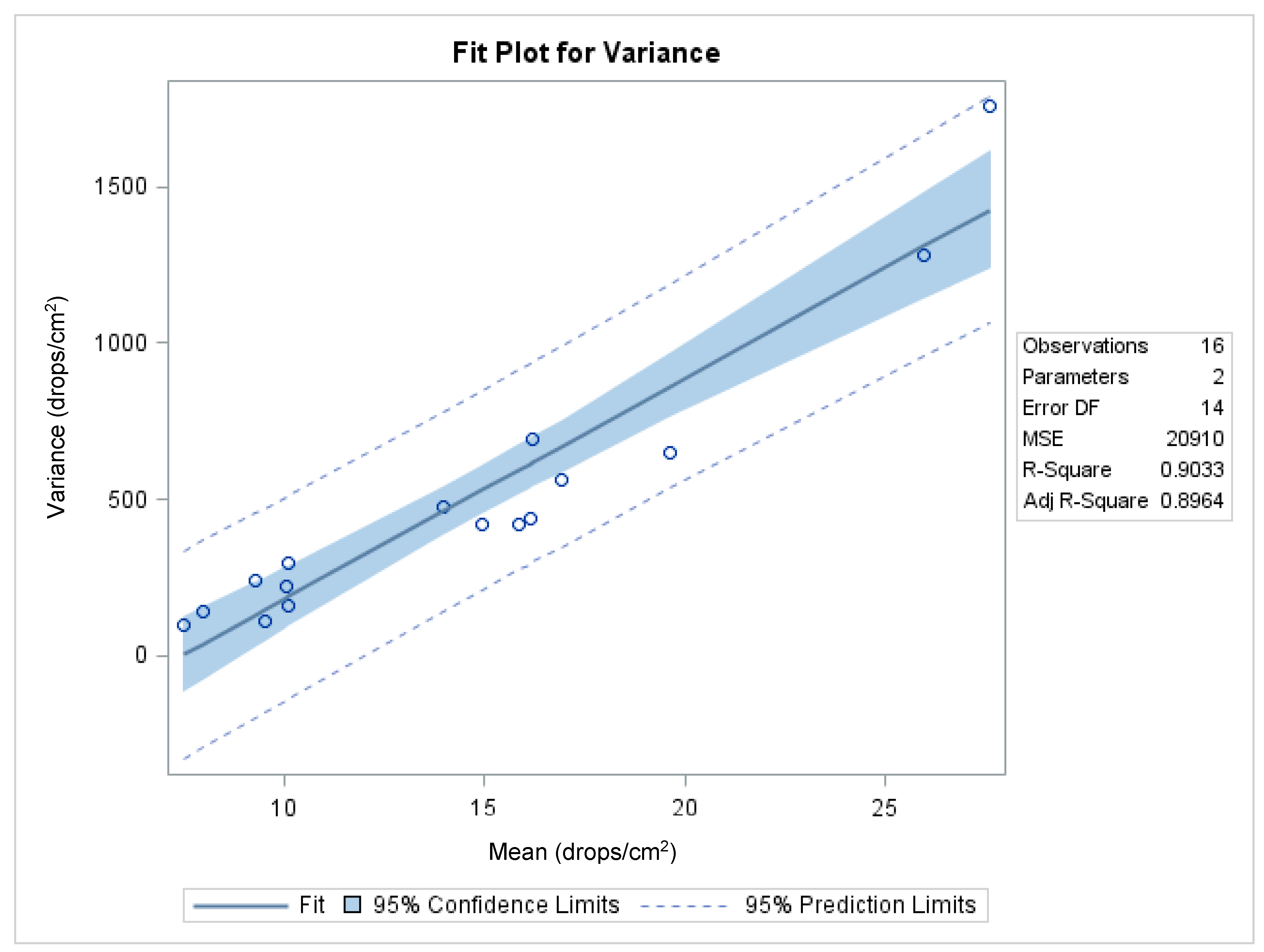
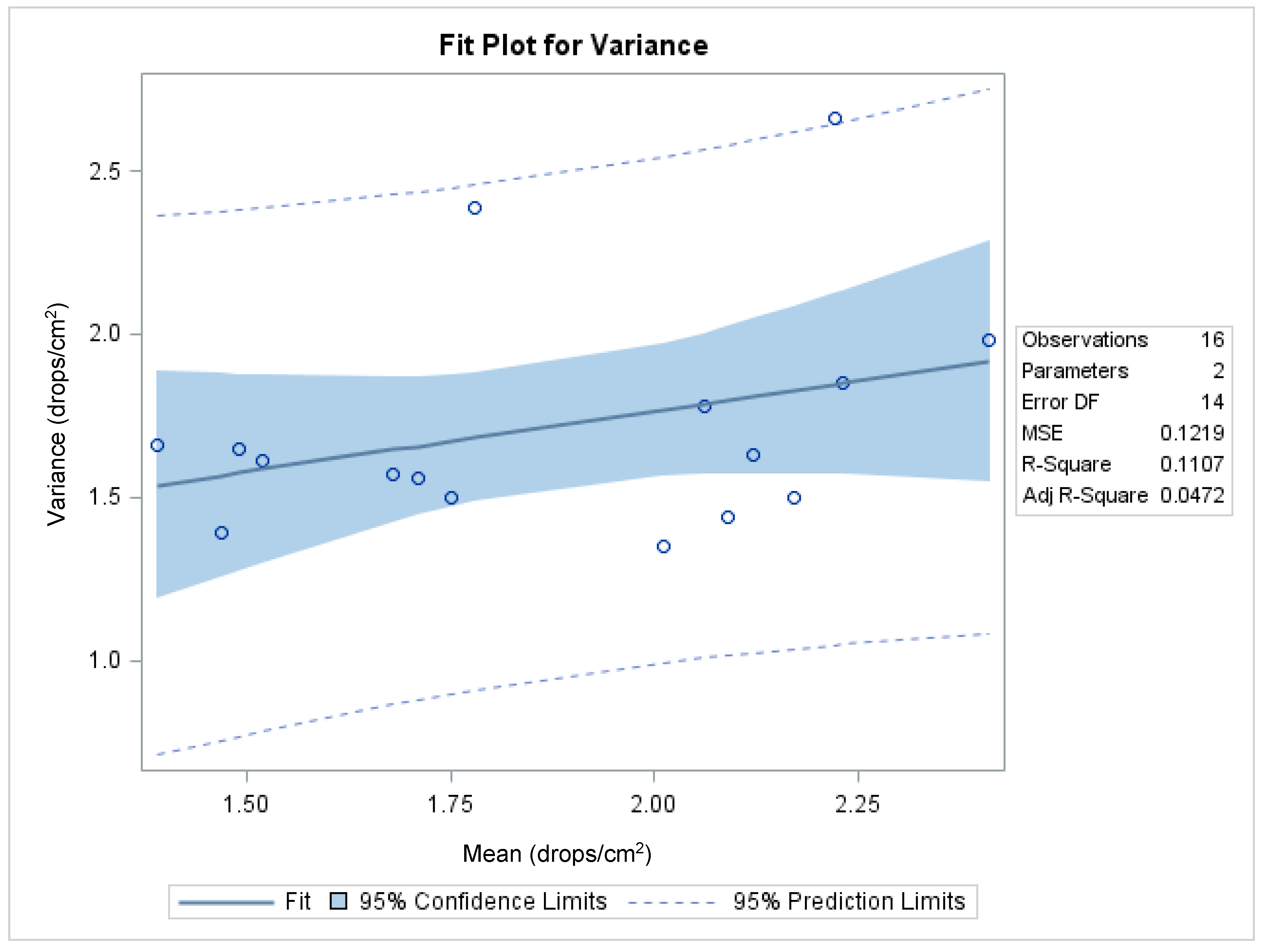
Figure 7 shows the functional relationship between the variance and the mean, with an R
2 of 0.9033 (
p < 0.0001; df = 14) and a highly significant slope coefficient (
b = 70.7;
t = 11.4;
p < 0.0001), which indicates that the data do not meet the assumptions of the analysis of variance. The adequacy of the transformation in stabilizing the variance was achieved following transformation by calculating the correlation coefficient between the two parameters (
Figure 8) as suggested by several researchers [
36,
37,
38,
39]. The coefficient of determination between the two parameters was 0.11, with a non-significant slope coefficient (
b = 0.37;
t = 1.32;
p > 0.21). The transformed fluorescent droplet density data were used for statistical analysis. All other data were analyzed without transformation. The analysis of variance of the data was conducted using the Proc GLIMMIX procedure (SAS) and least square means were separated using the lines option at
p < 0.05 when sample size was equal [
40]. The replicated block effects were not significant for any of the data discussed in this study.