Abstract
A graphene-based electrochemical biosensor was developed for the detection of matrix metalloproteinase 2 (MMP-2) endopeptidase, whose expression can be significantly related to the occurrence, metastasis, and prognosis of cancer. A specific anti-MMP-2 aptamer was successfully immobilized on the surface of electrochemically reduced graphene oxide via a pyrene-based linker, enabling the specific capture of MMP-2. The sensor was able to detect 1 ng mL−1, with an overall detection time of less than 20 min. Moreover, the aptamer-based biosensor showed good specificity toward different unspecific proteins.
1. Introduction
Matrix metalloproteinases (MMPs) are a family of extracellular Zn2+-dependent endopeptidases []. Due to their involvement in several aspects of cancer development, MMPs have been regarded as a hallmark of cancer []. In particular, MMP-2 is made by stromal cells, and it can be found on both stromal and cancer cell membranes []; therefore, its overexpression is significantly related to the occurrence, metastasis, and prognosis of cancer []. One of the electrochemical biosensing solutions for MMP-2 detection involves a sandwich assay performed on an electrode surface by using graphene-oxide micro-flakes functionalized with a detection antibody and horseradish peroxidase (HRP), resulting in a detection limit of 0.11 pg mL−1 []. Mishyn and co-workers developed an MMP-2 aptasensor with gold interdigitated electrodes (IDE) functionalized with CVD graphene, which was modified with an anti-MMP-2 aptamer. By using differential pulse voltammetry (DPV), a limit of detection of 100 pM (920 pg mL−1) was obtained [].
Here, we report the development of an electrochemical biosensing platform for the detection of MMP-2 based on graphene oxide-coated gold interdigitated electrodes and a specific anti-MMP2 aptamer.
2. Materials and Methods
Clean gold interdigitated electrodes (ED-IDE1-Au, Micrux, Gijón, Spain) were treated with APTES ((3-aminopropyl) triethoxysilane) (Sigma Aldrich, St. Louis, MO, USA) solution to activate the glass surface. A graphene oxide thin film was prepared via spin coating with subsequent electrochemical reduction to obtain a conductive reduced GO film. 1-pyrenebutyric acid N-hydroxysuccinimide ester (PBASE, Sigma Aldrich, USA) in N,N-dimethylformamide (DMF, Sigma Aldrich, USA) was incubated on the electrode to activate rGO sheets for the immobilization of the anti-MMP2 aptamer (Metabion AG, Planegg, Germany). Finally, 0.05% w/w BSA in water was incubated to block the remaining free surface. MMP-2 (pre-activated human, Sigma Aldrich, USA) was serially diluted from a stock solution to obtain a concentration range of 1 ng mL−1 to 100 ng mL−1 in 1× PBS. All electrochemical measurements were performed on a Biologic VMP 3e Multichannel potentiostat (Seyssinet-Pariset, France), using 5 mM of K3[Fe(CN)6]/K4[Fe(CN)6] (Sigma Aldrich, USA; Fluka, Barcelona, Spain) in 0.1 M KCl (Sigma Aldrich, USA).
3. Discussion
An rGO-based aptasensor was characterized electrochemically by CV and EIS after each step of the functionalization protocol. As shown in Figure 1A, a distinct oxidation peak was observed for bare gold and the rGO-functionalized electrode, while the peak drastically diminished after each step due to the presence of layered molecular structures that significantly reduced the electron and mass transfer between the electrode and redox probe []. As a proof of concept, the developed biosensor was tested to various MMP-2 concentrations prepared in 1× PBS. As presented in Figure 1B, the EIS data showed that the impedance increased as the concentration increased until 100 ng mL−1, where saturation was observed. The biosensor showed a good dynamical range between 1 and 50 ng mL−1. In addition, the aptasensor showed excellent selectivity with respect to other immuno- responsive proteins, such as IL-6 and IL-10.
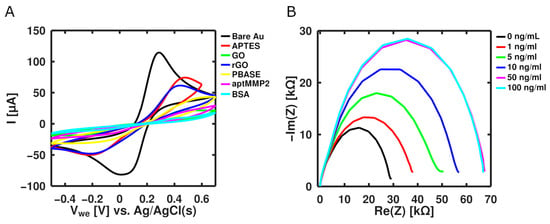
Figure 1.
(A) Graphene-based aptasensor assembly characterization via CV; (B) EIS spectra of different MMP-2 concentrations in 1× PBS.
Further studies on sensor reproducibility and repeatability, as well as detection in more complex environments, will be implemented to improve the current status of research on the electrochemical detection of MMP-2 using aptamers as a biorecognition element.
Author Contributions
Conceptualization and methodology, S.J., I.B. and S.S.; validation, S.J. and S.S.; formal analysis, S.J.; investigation, S.J.; resources, N.Ž.K. and P.E.; data curation, S.J. and I.B.; writing—original draft preparation, S.J.; writing—review and editing, I.B. and N.Ž.K.; visualization, S.J.; supervision, I.B. and N.Ž.K.; project administration, N.Ž.K. and P.E.; funding acquisition, N.Ž.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by EC Twinning project NANOFACTS (Networking Activities for Nanotechnology—Facilitated Cancer Theranostics), grant agreement No. 952259 (https://doi.org/10.3030/952259, accessed on 27 March 2023). IB acknowledges ANTARES project funded from European Union’s Horizon 2020 research and innovation programme, under grant agreement No. 664387 (https://doi.org/10.3030/739570, accessed on 27 March 2023).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nagase, H.; Woessner, J.F. Matrix Metalloproteinases. J. Biol. Chem. 1999, 274, 21491–21494. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Werb, Z. New functions for the matrix metalloproteinases in cancer progression. Nat. Rev. Cancer 2002, 2, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Chien, M.H.; Lee, W.J.; Yang, Y.C.; Tan, P.; Pan, K.F.; Liu, Y.C.; Tsai, H.C.; Hsu, C.H.; Wen, Y.C.; Hsiao, M.; et al. N-α-acetyltransferase 10 protein promotes metastasis by stabilizing matrix metalloproteinase-2 protein in human osteosarcomas. Cancer Lett. 2018, 443, 86–98. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Li, L.; Rana, R.K.; Zhu, J.J. Assembled gold nanoparticles on nitrogen-doped graphene for ultrasensitive electrochemical detection of matrix metalloproteinase-2. Carbon 2013, 61, 357–366. [Google Scholar] [CrossRef]
- Mishyn, V.; Aslan, M.; Hugo, A.; Rodrigues, T.; Happy, H.; Sanyal, R.; Knoll, W.; Baudoux, F.; Bouchiat, V.; Bilyy, R.O.; et al. Catch and release strategy of matrix metalloprotease aptamers via thiol–disulfide exchange reaction on a graphene based electrochemical sensor. Sens. Diagn. 2022, 1, 739–749. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, F.; Wang, Z.; Liang, Q. A graphene oxide-based label-free electrochemical aptasensor for the detection of alpha-fetoprotein. Biosens. Bioelectron. 2018, 112, 186–192. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).