A Phenylalanine Ammonia Lyase Capacitive Sensor for Phenylalanine Detection †
Abstract
1. Introduction
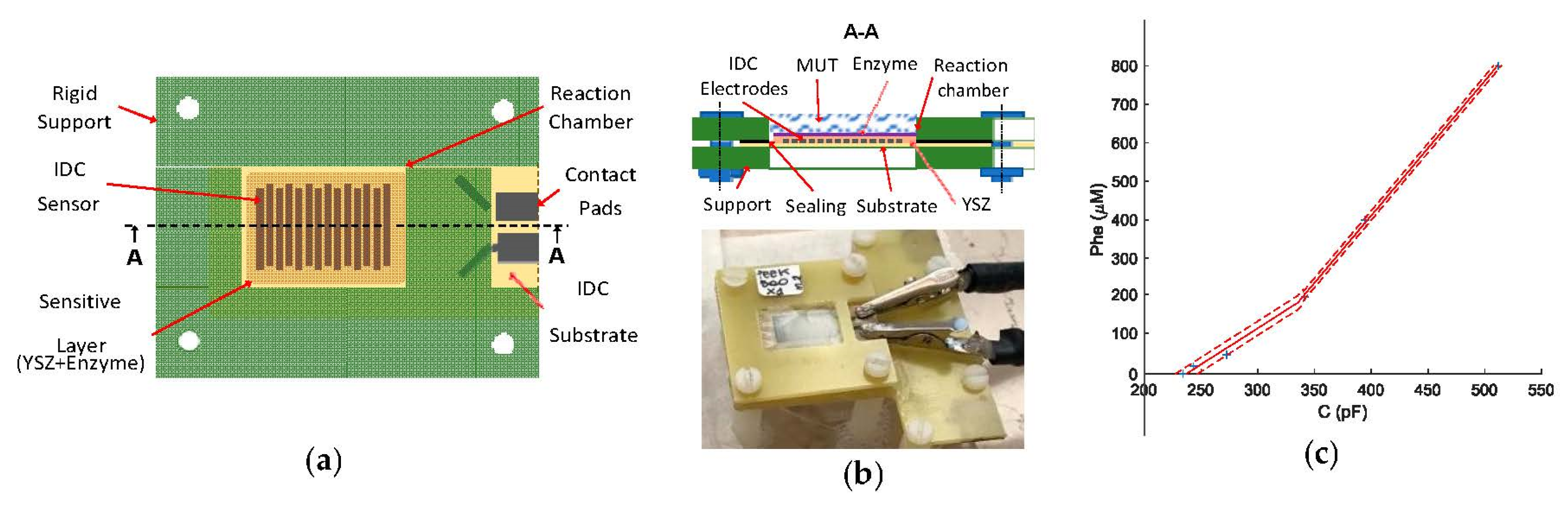
2. The Developed Device
3. Experimental Results
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blau, N.; Hennermann, J.B.; Langenbeck, U.; Lichter-Konecki, U. Diagnosis, classification, and genetics of phenylketonuria and tetrahydrobiopterin (BH4) deficiencies. Mol. Genet. Metab. 2011, 104, S2–S9. [Google Scholar] [CrossRef] [PubMed]
- da Silva, K.P.; Ptak, M.; Pizani, P.S.; Mendes Filho, J.; Melo, F.E.A.; Freire, P.T.C. Raman spectroscopy of L-phenylalanine nitric acid submitted to high pressure. Vib. Spectrosc. 2016, 85, 97–103. [Google Scholar] [CrossRef]
- Shen, W.C.; Shih, P.J.; Tsai, Y.C.; Hsu, C.C.; Dai, C.L. Low concentration ammonia gas sensors manufactured using the CMOSMEMS technique. Micromachines 2020, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Winquist, F.; Spetz, A.; Lundström, I.; Danielsson, B. Determination of ammonia in air and aqueous samples with a gas-sensitive semiconductor capacitor. Anal. Chim. Acta 1984, 164, 127–138. [Google Scholar] [CrossRef]
- Andò, B.; Baglio, S.; Castorina, S.; Graziani, S.; Messina, M.; Petralia, S.; Tondepu, S.V.G. A Capacitive Readout Strategy for Ammonia Detection: Design Flow, Modeling and Simulation. In Proceedings of the 2021 IEEE Sensors Applications Symposium (SAS), Sundsvall, Sweden, 23–25 August 2021; pp. 1–6. [Google Scholar]
- Andò, B.; Baglio, S.; Castorina, S.; Graziani, S.; Tondepu, S.V.G.; Petralia, S.; Messina, M.A.; Maugeri, L.; Neri, G.; Ferlazzo, A. A Capacitive Sensor, Exploiting a YSZ Functional Layer, for Ammonia Detection. IEEE Trans. Instrum. Meas. 2022, 71, 9505811. [Google Scholar] [CrossRef]
- Messina, M.A.; Meli, C.; Conoci, S.; Petralia, S. A facile method for urinary phenylalanine measurement on paper-based lab-on-chip for PKU therapy monitoring. Analyst 2017, 142, 4629–4632. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andò, B.; Castorina, S.; Maugeri, L.; Petralia, S.; Messina, M.A.; Ruggieri, M.; Neri, G.; Ferlazzo, A.; Sardini, E.; Serpelloni, M. A Phenylalanine Ammonia Lyase Capacitive Sensor for Phenylalanine Detection. Proceedings 2024, 97, 51. https://doi.org/10.3390/proceedings2024097051
Andò B, Castorina S, Maugeri L, Petralia S, Messina MA, Ruggieri M, Neri G, Ferlazzo A, Sardini E, Serpelloni M. A Phenylalanine Ammonia Lyase Capacitive Sensor for Phenylalanine Detection. Proceedings. 2024; 97(1):51. https://doi.org/10.3390/proceedings2024097051
Chicago/Turabian StyleAndò, Bruno, Salvatore Castorina, Ludovica Maugeri, Salvatore Petralia, Maria Anna Messina, Martino Ruggieri, Giovanni Neri, Angelo Ferlazzo, Emilio Sardini, and Mauro Serpelloni. 2024. "A Phenylalanine Ammonia Lyase Capacitive Sensor for Phenylalanine Detection" Proceedings 97, no. 1: 51. https://doi.org/10.3390/proceedings2024097051
APA StyleAndò, B., Castorina, S., Maugeri, L., Petralia, S., Messina, M. A., Ruggieri, M., Neri, G., Ferlazzo, A., Sardini, E., & Serpelloni, M. (2024). A Phenylalanine Ammonia Lyase Capacitive Sensor for Phenylalanine Detection. Proceedings, 97(1), 51. https://doi.org/10.3390/proceedings2024097051










