A Portable Gold-Nanoparticle-Enhanced Surface Plasmon Resonance Sensor for Highly Sensitive β-Bungarotoxin Quantification in Snake Poisoning Diagnosis †
Abstract
1. Introduction
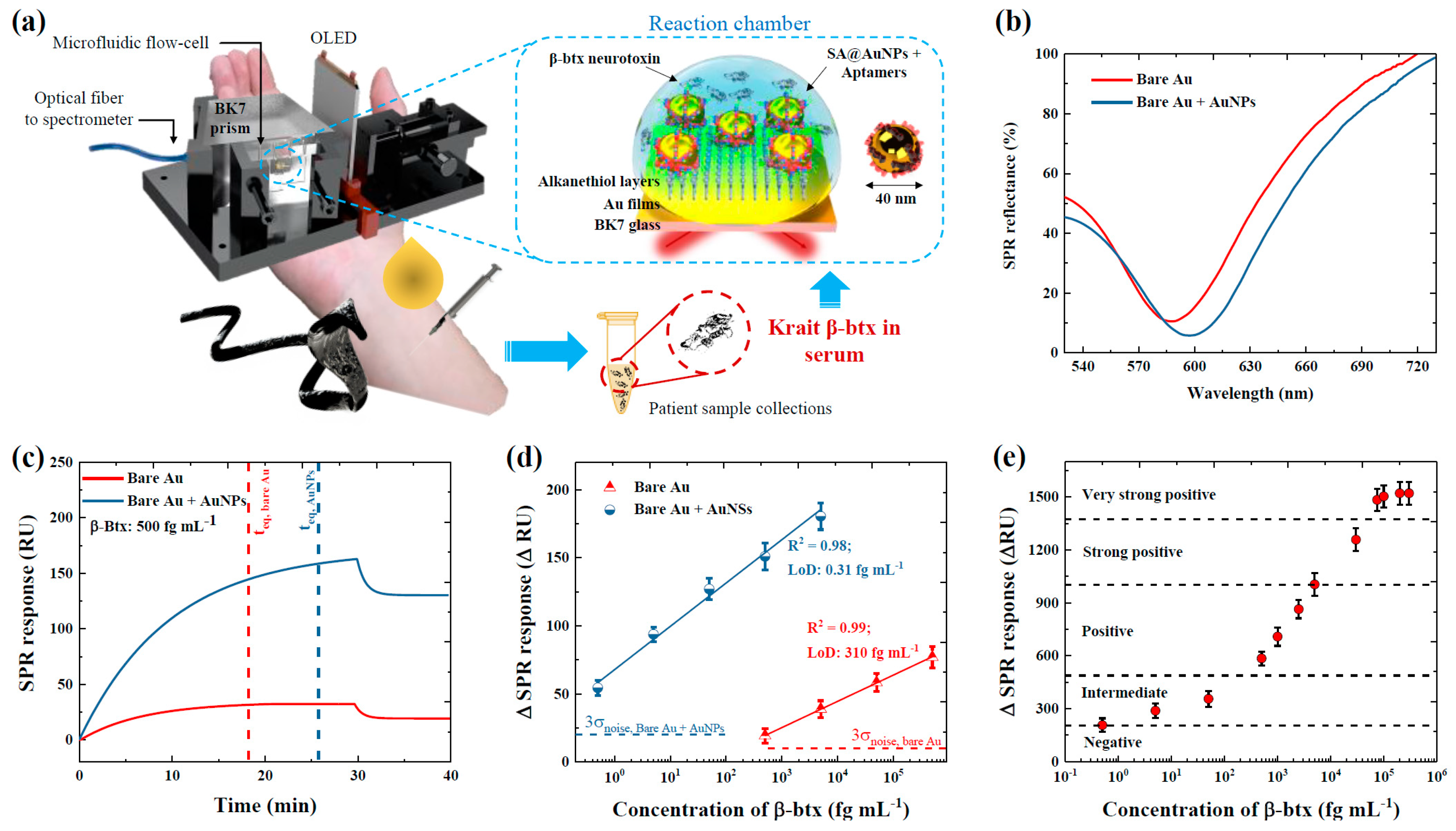
2. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Primers 2017, 3, 17063. [Google Scholar] [CrossRef] [PubMed]
- Puzari, U.; Mukherjee, A.K. Recent developments in diagnostic tools and bioanalytical methods for analysis of snake venom: A critical review. Anal. Chim. Acta 2020, 1137, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Masson, J.F. Surface Plasmon Resonance Clinical Biosensors for Medical Diagnostics. ACS Sens. 2017, 2, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Mandala, S.H.S.; Liu, T.-J.; Chen, C.-M.; Liu, K.-K.; Januar, M.; Chang, Y.-F.; Lai, C.-S.; Chang, K.-H.; Liu, K.-C. Enhanced Plasmonic Biosensor Utilizing Paired Antibody and Label-Free Fe3O4 Nanoparticles for Highly Sensitive and Selective Detection of Parkinson’s α-Synuclein in Serum. Biosensors 2021, 11, 402. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Wu, P.F.; Wu, T.T.; Chang, L.S. KChIP3: A binding protein for Taiwan banded krait β-bungarotoxin. Toxicon 2006, 47, 265–270. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mandala, S.H.S.; Januar, M.; Liu, C.-C.; Yu, J.-S.; Liu, K.-C. A Portable Gold-Nanoparticle-Enhanced Surface Plasmon Resonance Sensor for Highly Sensitive β-Bungarotoxin Quantification in Snake Poisoning Diagnosis. Proceedings 2024, 97, 229. https://doi.org/10.3390/proceedings2024097229
Mandala SHS, Januar M, Liu C-C, Yu J-S, Liu K-C. A Portable Gold-Nanoparticle-Enhanced Surface Plasmon Resonance Sensor for Highly Sensitive β-Bungarotoxin Quantification in Snake Poisoning Diagnosis. Proceedings. 2024; 97(1):229. https://doi.org/10.3390/proceedings2024097229
Chicago/Turabian StyleMandala, Samuel Husin Surya, Mochamad Januar, Chien-Chun Liu, Jau-Song Yu, and Kou-Chen Liu. 2024. "A Portable Gold-Nanoparticle-Enhanced Surface Plasmon Resonance Sensor for Highly Sensitive β-Bungarotoxin Quantification in Snake Poisoning Diagnosis" Proceedings 97, no. 1: 229. https://doi.org/10.3390/proceedings2024097229
APA StyleMandala, S. H. S., Januar, M., Liu, C.-C., Yu, J.-S., & Liu, K.-C. (2024). A Portable Gold-Nanoparticle-Enhanced Surface Plasmon Resonance Sensor for Highly Sensitive β-Bungarotoxin Quantification in Snake Poisoning Diagnosis. Proceedings, 97(1), 229. https://doi.org/10.3390/proceedings2024097229





