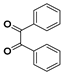
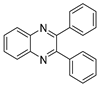
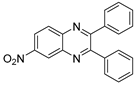
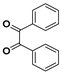
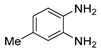
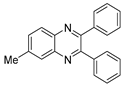
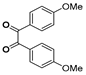
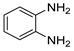
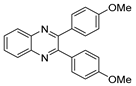
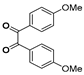
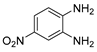
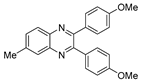
g-C3N4/Ni Nanocomposite: An Efficient and Eco-Friendly Recyclable Catalyst for the Synthesis of Quinoxalines †
Abstract
:1. Introduction
2. Experimental
2.1. General
2.2. Preparation of Bulk g-C3N4
2.3. Preparation of g-C3N4/Ni Nanocomposite
2.4. General Synthesis of Quinoxaline Derivatives
3. Results and Discussion
4. Conclusions
Acknowledgments
References
- Refaat, H.; Badran, M.; Botros, S.; El-Gendy, A.; Abdou, N.; El-Assi, H.; Salem, A. g-C3N4/Ni nanocomposite: An efficient and eco-friendly recyclable catalyst for the synthesis of quinoxalines. Bull. Pharm. Sci. 2001. [Google Scholar] [CrossRef]
- Hazeldine, S.T.; Polin, L.; Kushner, J.; White, K.; Bouregeois, N.M.; Crantz, B.; Palomino, E.; Corbett, T.H.; Horwitz, J.P. II. Synthesis and biological evaluation of some bioisosteres and congeners of the antitumor agent, 2-(4-[(7-chloro-2-quinoxalinyl)oxy]phenoxy)propionic acid (XK469). J. Med. Chem. 2002, 45, 3130–3137. [Google Scholar] [CrossRef] [PubMed]
- Sakata, G.; Makino, K.; Kurasawa, Y. Reaction of Quinoxalin-2-ones with Difluorocarbene. J. Fluorine. Chem. 1992, 59, 417–422. [Google Scholar]
- Dailey, S.; Feast, W.J.; Peace, R.J.; Sage, I.C.; Till, S.; Wood, E.L. Synthesis and device characterisation of side-chain polymer electron transport materials for organic semiconductor applications. J. Mater. Chem. 2001, 11, 2238–2243. [Google Scholar] [CrossRef]
- Sonawane, N.; Rangnekar, D.J. An efficient synthesis of quinoxalines catalyzed by monoammonium salt of 12-tungstophosphoric acid. Heterocycl. Chem. 2002, 39, 303–308. [Google Scholar] [CrossRef]
- Sessler, J.L.; Maeda, H.; Mizuno, T.; Lynch, V.M.; Furuta, H. Anion Recognition in Supramolecular Chemistry. JACS 2002, 124, 13474–13479. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.J.; Taylor, E.C.; Ellman, J.A. Quinoxalines, Spplement 2; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Gupta, P.; Paul, S. Solid Acids: Green Alternatives for Acid Catalysis. Catal. Today 2014, 236, 153–170. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xiao, J.; Wang, H.; Shao, M. Carbon-Based Electrocatalysts for Hydrogen and Oxygen Evolution Reactions. ACS Catal. 2017, 7, 7855–7865. [Google Scholar] [CrossRef]
- Habibi-Yangjeh, A.; Akhundi, A.J. Novel ternary g-C3N4/Fe3O4/Ag2CrO4 nanocomposites: magnetically separable and visible-light-driven photocatalysts for degradation of water pollutants. Mol. Catal. A Chem. 2016, 415, 122–130. [Google Scholar] [CrossRef]
- Sun, J.; Fu, Y.; He, G.; Sun, X.; Wang, X. Green Suzuki–Miyaura coupling reaction catalyzed by palladium nanoparticles supported on graphitic carbon nitride. Appl. Catal. B 2015, 165, 661–667. [Google Scholar] [CrossRef]
- Hasaninejad, A.; Zare, A.; Mohammadizadeh, M.R.; Shekouhya, M. Oxalic acid as an efficient, cheap, and reusable catalyst for the preparation of quinoxalines via condensation of 1, 2-diamines with α-diketones at room temperature. Arkivoc 2008, 13, 28–35. [Google Scholar] [CrossRef]
- Jafarpour, M.; Rezaeifard, A.; Ghahramaninezhad, M.; Tabibi, T. Reusable α-MoO 3 nanobelts catalyzes the green and heterogeneous condensation of 1, 2-diamines with carbonyl compounds. New J. Chem. 2013, 37, 2087–2095. [Google Scholar] [CrossRef]
- Harsha, K.B.; Rangappa, K.S. One-step approach for the synthesis of functionalized quinoxalines mediated by T3P®–DMSO or T3P® via a tandem oxidation–condensation or condensation reaction. RSC Adv. 2016, 6, 57154–57162. [Google Scholar] [CrossRef]
- Daragahi, S.A.H.; Mohebat, R.; Mosslemin, M.H. Green and Eco-Friendly Synthesis of Quinoxalines by Brönsted Acidic Ionic Liquid Supported on Nano-SiO2 under Solvent-Free Conditions. Org. Prep. Proced. Int. 2018, 50, 301–313. [Google Scholar] [CrossRef]
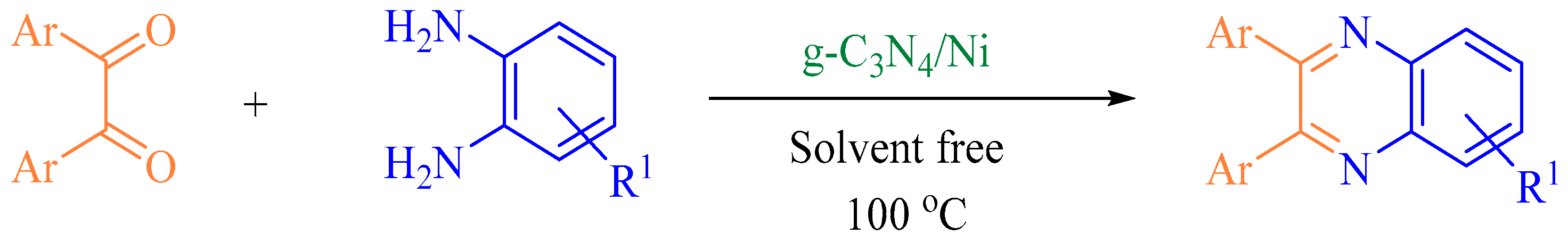
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rashidizadeh, A.; Ghafuri, H. g-C3N4/Ni Nanocomposite: An Efficient and Eco-Friendly Recyclable Catalyst for the Synthesis of Quinoxalines. Proceedings 2019, 9, 49. https://doi.org/10.3390/ecsoc-22-05651
Rashidizadeh A, Ghafuri H. g-C3N4/Ni Nanocomposite: An Efficient and Eco-Friendly Recyclable Catalyst for the Synthesis of Quinoxalines. Proceedings. 2019; 9(1):49. https://doi.org/10.3390/ecsoc-22-05651
Chicago/Turabian StyleRashidizadeh, Afsaneh, and Hossein Ghafuri. 2019. "g-C3N4/Ni Nanocomposite: An Efficient and Eco-Friendly Recyclable Catalyst for the Synthesis of Quinoxalines" Proceedings 9, no. 1: 49. https://doi.org/10.3390/ecsoc-22-05651
APA StyleRashidizadeh, A., & Ghafuri, H. (2019). g-C3N4/Ni Nanocomposite: An Efficient and Eco-Friendly Recyclable Catalyst for the Synthesis of Quinoxalines. Proceedings, 9(1), 49. https://doi.org/10.3390/ecsoc-22-05651