Published: 14 November 2018
1. Introduction
In small quantities, Cu
2+ is an essential nutrient required to maintain health. Nevertheless, at high concentration, it is toxic for living organisms, and thus the early detection of Cu
2+ contamination in the environment is desirable [
1]. Fluorogenic chemosensors are attractive and versatile tools for analytical sensing because of their high sensibility, fast response time, and technical simplicity. In this sense, we have recently reported the design and synthesis of a new chemosensor for the determination of Cu
2+ [
2].
Because of this area of investigation, the need to develop new chemosensors composed of ligands supported in solid matrices appeared. In addition, mesoporous material such as silicas, which exhibit ordered pore systems and uniform pore diameters, have shown great potential for sensing applications in recent years [
3]. With mesoporous SiO
2, morphological control grants it versatility in the methods of deployment, whether as bulk powders, monoliths, thin films, or embedded in coatings. High surface areas and pore sizes greater than 20 Å make them an effective matrix to support ligands [
4].
Considering that the more rigid structure of a chemosensor tends to generate changes in fluorescence, mesoporous silica was used as a matrix to embed the ligand, to maintain its properties as a sensor [
5].
In the present work, we synthetized a composite formed by a double benzothiazole hydrazone derived from 5,5’-bis-vanillin and mesoporous SiO2. We found that this material has a high sensitivity and selectivity to detect Cu2+ over other cations.
2. Experimental
2.1. Synthesis of Mesoporous Silica
Mesoporous silica was prepared by using Pluronic P123 triblock copolymer surfactant as a template in acid media [
6]. First, 4 g of Pluronic P123 was dissolved at room temperature in a 350 mL of 3.1 M aqueous HCl solution. Then, 10 g of Polyetilenglicol 400 was added and the resulting solution was slowly stirred at 35 °C until the solution became clear. Subsequently, 22.5 mL of Tetraethyl orthosilicate was then added to the solution and the resulting mixture was vigorously stirred at 40 °C for 24 h. Subsequently, the container was transferred to a microwave oven and kept at 100 °C for 12 h under static conditions. The resultant product was filtered, washed with water, and dried at 80 °C overnight.
2.2. Synthesis of Ligand
The
bis-vanillin (BVA) initial was prepared from the oxidative coupling of vanillin with sodium persulphate and iron sulphate [
7]. First, 3.55 g of vanillin (23.33 mmol) and 0.135 g of ferrous sulfate (0.89 mmol) were dissolved in 240 mL of distilled water and stirred for 10 min. Then, 3.38 g of potassium persulfate (12.51 mmol) was added and the reaction mixture was maintained for 5 days at 70 °C. The solid was filtered and dried.
In a second state, the byphenyl hydrazone derived from
bis-vanillin (L) was synthesized [
7]. A solution of BVA (0.66 mmol) in ethanol was added to a solution composed of 2-hydrazinobenzothiazole (1.32 mmol), sodium acetate (22.55 mmol) and 10 mL of distilled water. Then the system was maintained under reflux for 4 days. The compound obtained was isolated by filtration and purified by recrystallization.
2.3. Synthesis of Byphenyl Hydrazone@SiO2 Compositer
The byphenyl hydrazone@SiO2 chemosensor (L@SiO2) was prepared using an Anton Paar Monowave 300. A mixture of 0.15 g of L and 1.5 g of mesoporous SiO2 was put in the vial an a solution of ethanol:water 50:50 (18 mL) was added. Then the mixture was heating in a microwave oven and kept at 70 °C at 1200 rpm for about 20 min. After a slow cooling of the mixture at room temperature, the resulting material was dried at 80 °C overnight.
2.4. Characterization of Precursor Materials and Composite
The geometry optimization of L molecule was performed with Gaussian 09 software, using DFT level of theory with the gradient corrected by Becke’s three parameters hybrid exchange in combination with the correlation of Lee, Yang and Parr (B3LYP) and 6-31G(d,p) set of base funtions.
1H and 13C NMR spectra were recorded using a Bruker DPX-300 (300 MHz) spectrometer. Nitrogen adsorption-desorption isotherms were measured at −196 °C on a NOVA-1000 Quantachrome. The samples were treated at 100 °C in the degassing port of the adsorption analyzer prior to testing. Specific surface areas were evaluated using the Brunauer, Emmett and Teller (BET) method, while pore size distributions were calculated using the Barret-Joyner-Halenda (BJH) algorithm on the adsorption branches of the isotherms.
The structural properties of materials were determined by X-ray diffraction (XRD) using a Shimadzu instrument XD-D1 equipped with Cu KR radiation and a Ni filter.
FTIR tests were performed on a Shimadzu FTIR Prestige-21 spectrophotometer in the region from 4000 to 1000 cm−1. The samples were mixed with KBr (1% wt) and then pressed. The UV absorption measurements were carried out on a Perkin Elmer Lambda 20 spectrophotometer. Fluorescence studies were performed on a Perkin Elmer F7000 spectrofluorometer. Spectrophotometric spectra were performed from a suspension of the sample in ethanol/water (50:50, v/v) and then brought into a quartz cell for measurement.
3. Results and discussion
The ligand L was obtained with excellent yield (95%). Its
1H and
13C NMR spectroscopic data were consistent with those reported in the bibliography and previous synthesis of our group [
7].
N
2 adsorption/desorption isotherms at −196 °C of SiO
2 and L@SiO
2 were determined to study their textural properties and results are summarized in
Table 1. Materials showed N
2 adsorption/desorption curves corresponding to type IV isotherms. The embedding of L into SiO
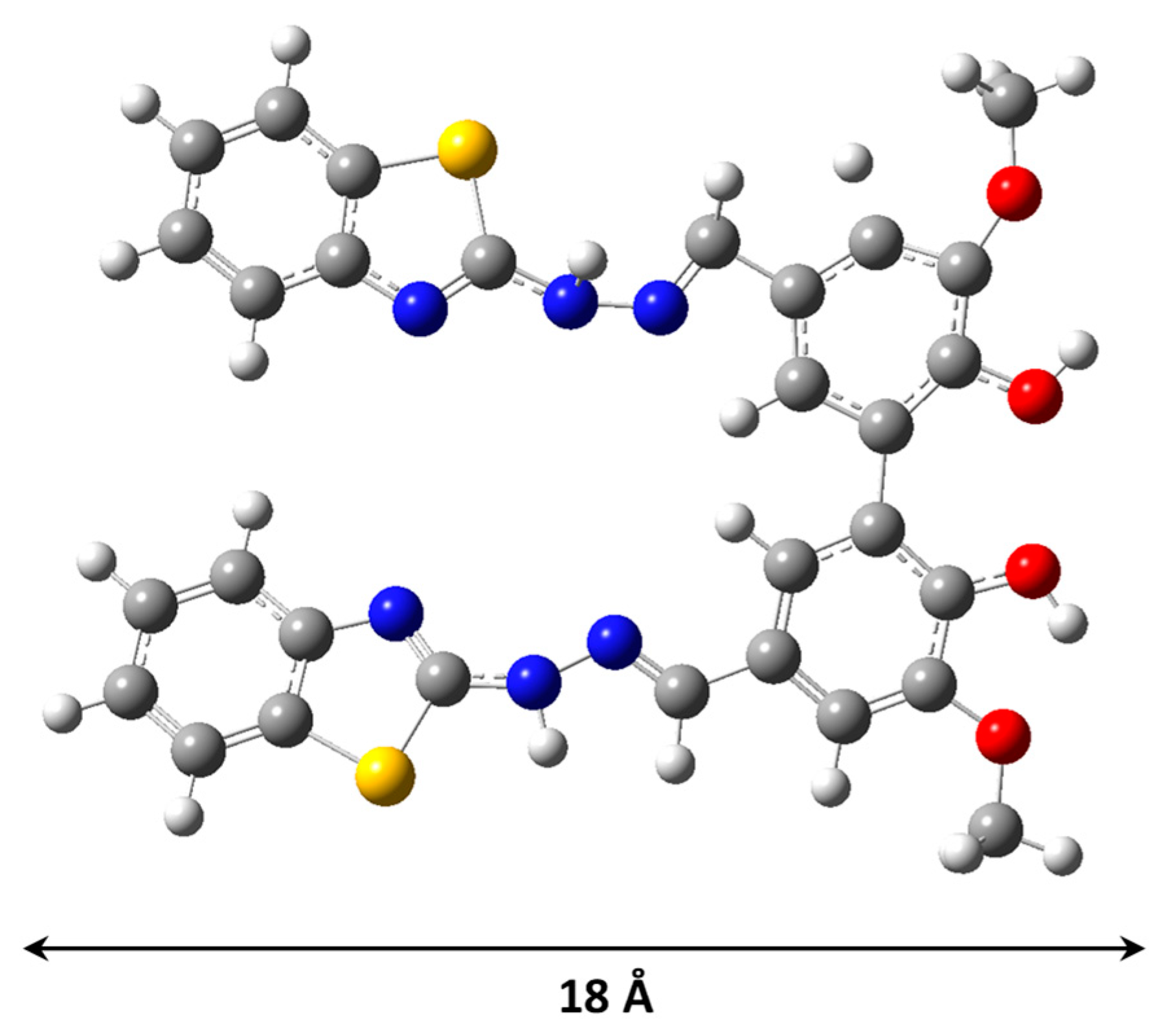
2 caused a reduction of specific surface area and a slight increase of average pore diameter while the pore volume is almost unchanged. The optimized geometrical structure of the ligand is shown in
Figure 1. From this geometry, the values of the molecular diameter (MD) obtained was 18 Å. Considering this value is possible to understand that the L molecules can be easily located within the pores of the SiO
2. The changes of textural parameters can be interpreted as that ligand molecules of the within the cannels of the silica hinder the adsorption of nitrogen.
The structural analysis by XRD of SiO2, the ligand and L@SiO2 composite presented amorphous structure, indicating that they are non-crystalline materials. The analysis of FTIR spectra of mesoporous SiO2 presented a peak near 1635 cm−1, mainly resulting from the bending vibration of absorbed H2O; and the typical Si-O-Si bands appear in OH bending region as three peaks: one broad and strong peak centered at 1084 cm−1; two narrow and relatively weak peaks near 850 and 465 cm−1, associated with the condensed silica network. Silanol groups of silica give the characteristic band at 964 cm−1, but the embedding of ligand made all peaks disappear, indicating the strong interaction between silanol groups of silica with molecules of ligand present into SiO2 pores.
To explore cromogenic and fluorogenic properties of these material, studies of UV-Vis and fluorescence spectroscopy of L and L@SiO2 were carried out. The selectivity of L for Cu2+, i.e., the sensing ability for L toward different cations evaluated by UV-Vis spectroscopy, showed a UV band to 460 nm only with Cu2+. Whereas, in the same conditions the other cations did not show any response. The initial solutions with L and L@SiO2 were colorless and turned red after addition of 1 equivalent of Cu2+.
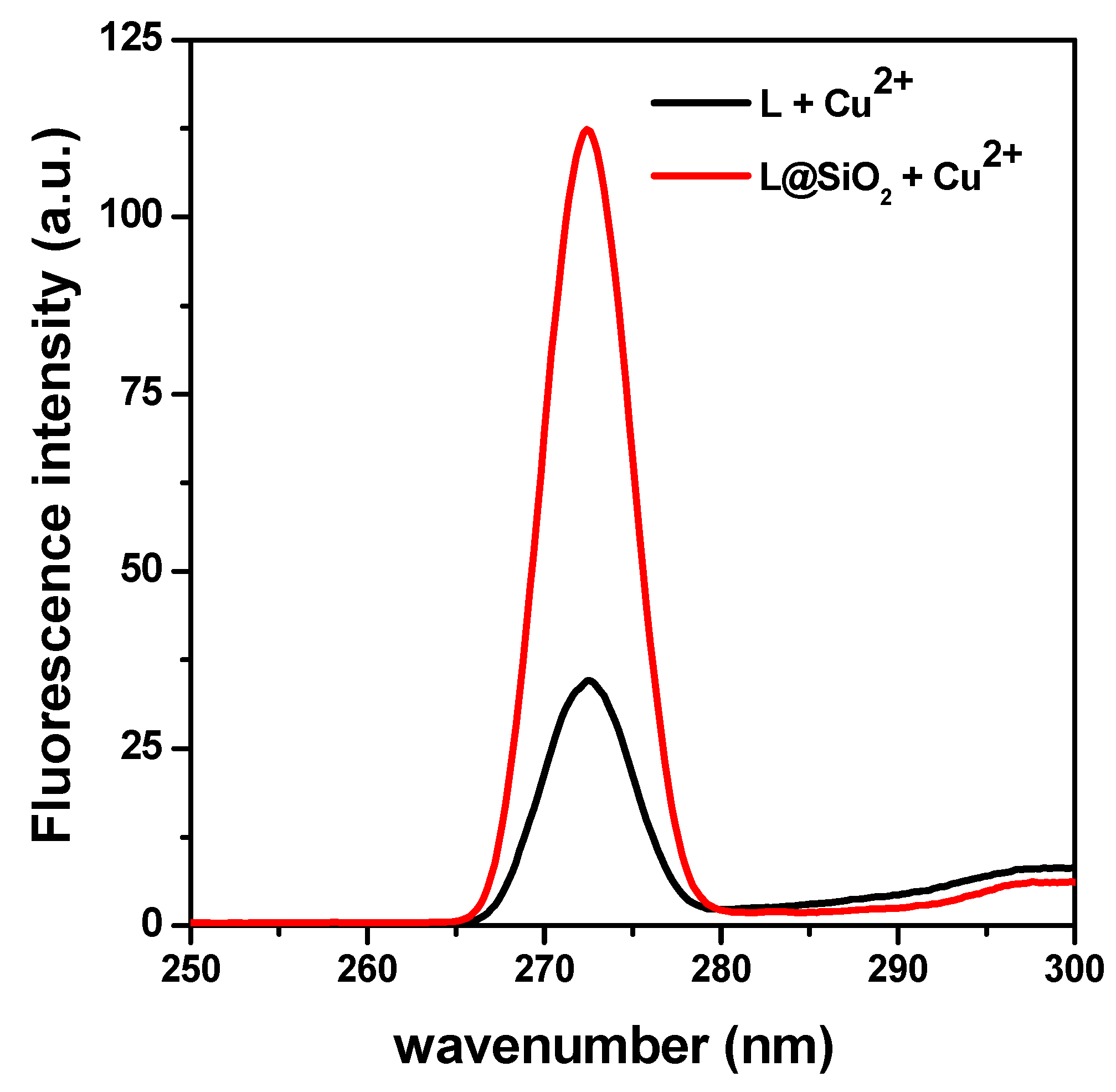
Similar study was carried out by fluorescence of spectroscopy. In the presence of Cu2+, mesoporous silica itself showed no fluorescence, whereas L and L@SiO2 samples showed an excitation band at 272 nm (under emission wavelength at 545 nm). The luminance phenomena of the L-Cu2+ complex is caused by the π electron conjugate system present in these molecules.
The L@SiO
2 composite gave a sensitive fluorescence response, to the same wavelength as the ligand free (
Figure 2), but the fluorescence intensity of the complex produced by the interaction of the composite with Cu
2+ is greater than that produced by the ligand. This would be because there is a greater dispersion of the ligand in the pores of the mesoporous silica, which is released under the test conditions forming the Li-Cu
2+ complex.
In conclusion, results suggest weak interactions of electrostatic nature (hydrogen bond) that connect ligand molecules with Si-OH groups present on the wall of mesoporous silica. These interactions do not cause chemical changes in the ligand and this would be widely dispersed in the silica matrix.
4. Conclusions
In summary, a novel L-SiO2 composite has been prepared by a process assisted by microwave. The physicochemical characterization of these materials confirmed the successful immobilization of the ligand into the mesoporous silica by weak interactions such as hydrogen bonds that connect the ligand molecules with the wall of SBA-15 without produce chemical changes. The spectroscopic tests evidenced the excellent performance of the L-SiO2 composite to be used as sensitive chemosensor of Cu2+.