Synthesis and Characterization of Various Amino Acid Derived Thiohydantoins †
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General
3.2. General Procedure for the Preparation of the Amino Acid Methyl Esters 2a–k
3.3. General Procedure for the Preparation of the Amino Acid Derived 2-Thiohydantoins 3a–k
4. Conclusions
Acknowledgments
References
- Ware, E. The chemistry of the hydantoins. Chem. Rev. 1950, 46, 403–470. [Google Scholar] [CrossRef] [PubMed]
- Konnert, L.; Lamaty, F.; Martinez, J.; Colacino, E. Recent advances in the synthesis of hydantoins: The state of the art of a valuable scaffold. Chem. Rev. 2017, 117, 13757–13809. [Google Scholar] [CrossRef] [PubMed]
- Bucherer, H.T.; Lieb, V.A. Über die bildung substituierter hydantoine aus aldehyden und ketonen. Synthese von hydantoinen. J. Prakt. Chem. 1934, 141, 5–43. [Google Scholar] [CrossRef]
- Heintz, W. Ueber ein Aethylderivat des Hydatoïns und die Bildung der Hydantoïnsäure aus Glycocoll. Justus Liebigs Annalen der Chemie 1865, 133, 65–74. [Google Scholar] [CrossRef]
- Marckwald, W.; Neumark, M.; Stelzner, R. Ueber Thiohydantoine und von diesen derivirende Basen. Gesellschaft 1891, 24, 3278–3298. [Google Scholar] [CrossRef]
- Ogawa, J.; Shimizu, S. Diversity and versatility of microbial hydantoin-transforming enzymes. J. Mol. Catal. B Enzym. 1997, 2, 163–176. [Google Scholar] [CrossRef]
- Trišović, N.P.; Ušćumlić, G.S.; Petrović, S.D. Hydantoins: Synthesis, properties and anticonvulsant activity. Hemijska Industrija 2009, 63, 17–31. [Google Scholar] [CrossRef]
- Rajic, Z.; Zorc, B.; Raic-Malic, S.; Ester, K.; Kralj, M.; Pavelic, K.; Balzarini, J.; de Clercq, E.; Mintas, M. Hydantoin Derivatives of L- and D-amino acids: Synthesis and Evaluation of Their Antiviral and Antitumoral Activity. Molecules 2006, 11, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, R.; Jabeen, R.; Zia-ul-Haq, M.; Nadeem, H.; Duddeck, H.; Verspohl, E.J. Chiral aryl sulfonyl Hydantoins as hypoglycemic agents. Z. Naturforsch. 2000, 55b, 203–207. [Google Scholar] [CrossRef]
- Reyes, S.; Burgess, K. On formation of thiohydantoins from amino acids under acylation conditions. J. Org. Chem. 2006, 71, 2507–2509. [Google Scholar] [CrossRef] [PubMed]
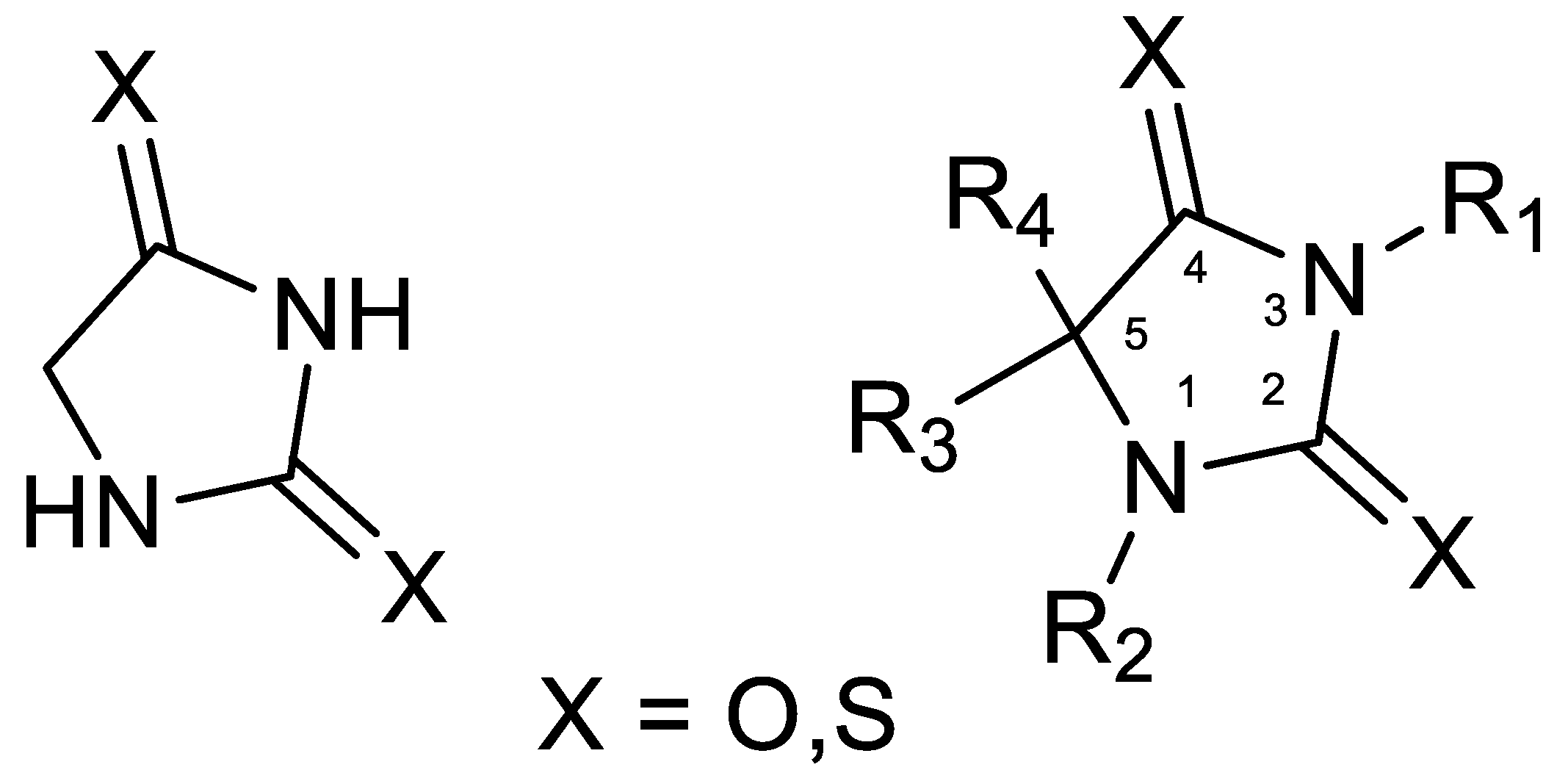
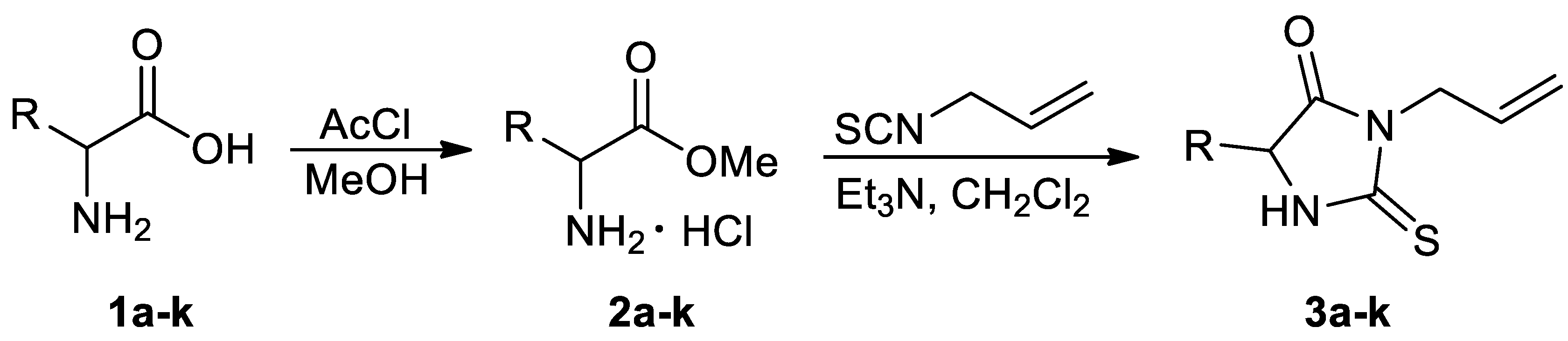

| Entry | Substrate | Product | Yield (%) |
|---|---|---|---|
| a | 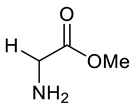 | 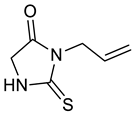 | 60 |
| b |  |  | 51 |
| c |  | 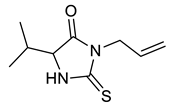 | 81 |
| d | 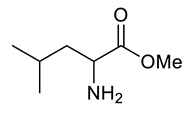 | 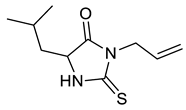 | 84 |
| e | 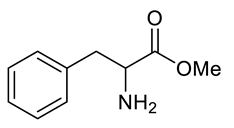 | 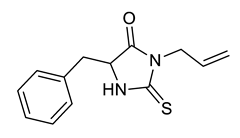 | 81 |
| f |  | 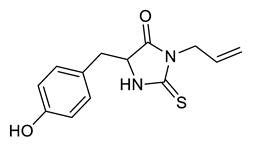 | 51 |
| g | 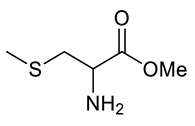 |  | 86 |
| h | 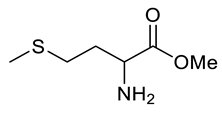 |  | 82 |
| i | 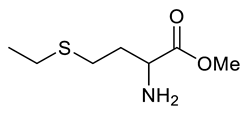 | 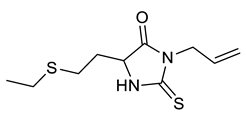 | 92 |
| j | 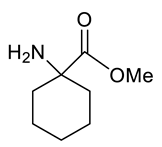 | 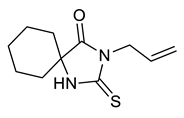 | 90 |
| k |  |  | 54 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stanić, P.; Živković, M.; Šmit, B. Synthesis and Characterization of Various Amino Acid Derived Thiohydantoins. Proceedings 2019, 9, 37. https://doi.org/10.3390/ecsoc-22-05690
Stanić P, Živković M, Šmit B. Synthesis and Characterization of Various Amino Acid Derived Thiohydantoins. Proceedings. 2019; 9(1):37. https://doi.org/10.3390/ecsoc-22-05690
Chicago/Turabian StyleStanić, Petar, Marija Živković, and Biljana Šmit. 2019. "Synthesis and Characterization of Various Amino Acid Derived Thiohydantoins" Proceedings 9, no. 1: 37. https://doi.org/10.3390/ecsoc-22-05690
APA StyleStanić, P., Živković, M., & Šmit, B. (2019). Synthesis and Characterization of Various Amino Acid Derived Thiohydantoins. Proceedings, 9(1), 37. https://doi.org/10.3390/ecsoc-22-05690





