Our Experience of Using Thermally Recycled Silica Gel in a Teaching and Small Research Laboratory Setting †
Abstract
1. Introduction
2. Material and Methods
3. Experimental
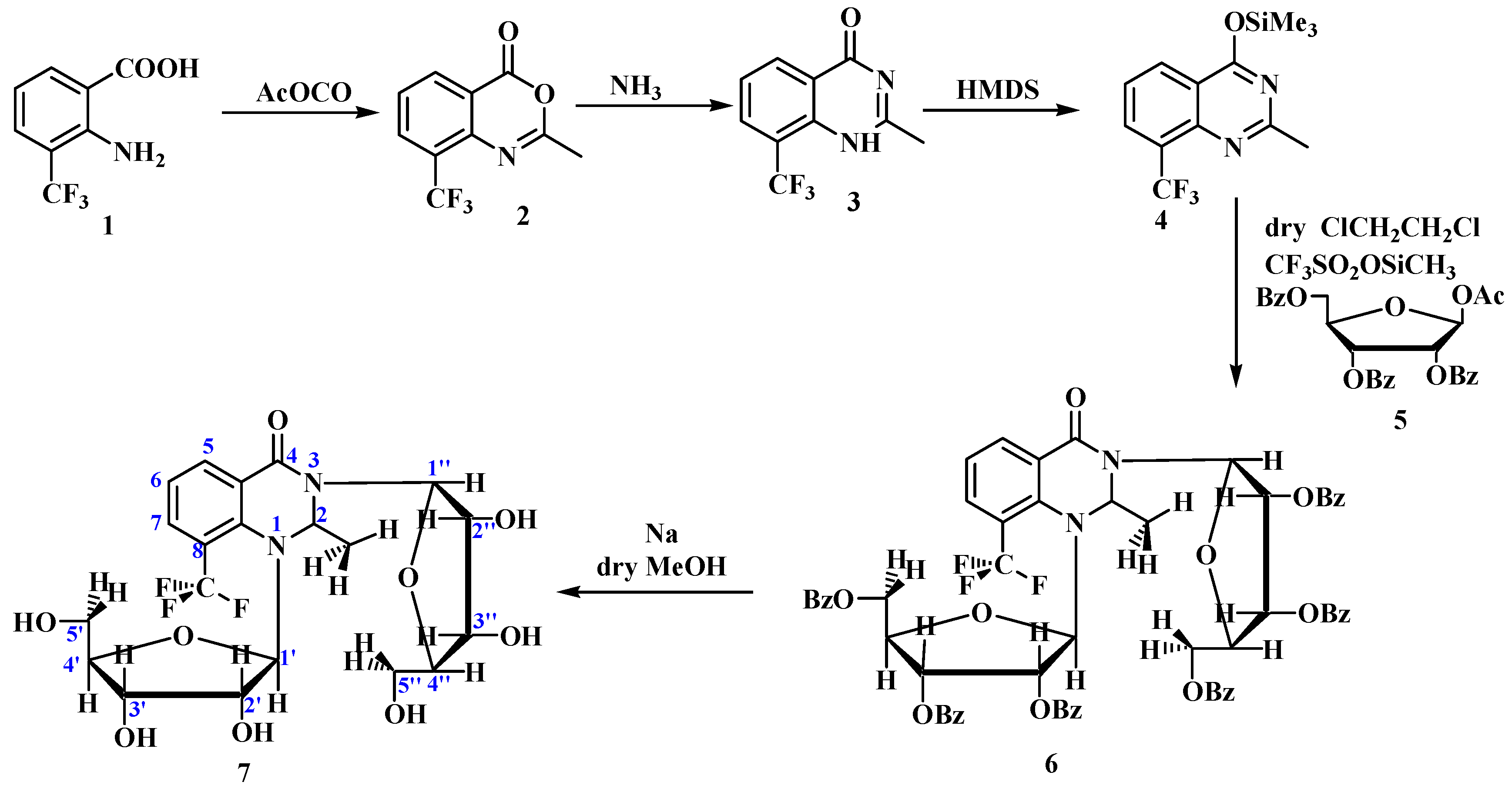
- Synthesis of 2-Methyl-8-(Trifluoromethyl)benzo[2,3-d] 4-Oxazinone 2: Compound 2 was prepared via refluxing 2-amino-3-(trifluoromethyl) benzoic acid 1 (3 g, 0.015 mol) with an acetic anhydride for 1 h. The residue was evaporated and washed several times with petroleum ether, then filtered and dried. Yield 2.64 g, (79.04%); m.p. 132 °C.
- Synthesis of of 1H-2-Methyl-8-(Trifluoromethyl)-4-Quinazolinone 3: Compound 3 was prepared by refluxing 2.6 g (0.01 mol) of compound 2 with 10 mL of ammonia, refluxing for 6 h, cooling, and treating with a few drops of acetic acid. Yield, 2.3 g (92%); m.p. 232–236 °C; 1HNMR CDCl3: 11.75 (s, 1H) NH; 8.48 (d, 1H; J = 6.8 Hz) H5; 8.09 (d, 1H; J = 7.65 Hz) H7; 7.52 (t, 1H) H6; 2.63 (s, 3H) CH3. 13CNMR CDCl3: 163.30, 154.18, 147.15, 132.94, 130.38, 125.33, 124.10, 122.82, 121.54, 22.59; Mass: M+ = 229.05(100%), 218.21, 209.05, 155.08, 151.03. Formula. C10H7F3N2O; M.wt: 228.17.
- Ribosylation of 1H-8-(Trifluoromethyl)-2-Methyl-4-Quinazolinone: Synthesis of 1,3-bis-(2,3,5-tri-O-benzoyl-β-d-Ribofuranosyl)-8-(Trifluoromethyl)-2-Methyl-4-Quinazolinone 6. 1H-8-(trifluoromethyl)-2-methyl-4-quinazolinone 3 (0.01 mol) and dry hexamethyldisilazane (20 mL) was heated under reflux for 24 h with a catalytic amount of ammonium sulfate. It was evaporated to dryness under anhydrous condition to give the silylated derivative 4, which was directly added (40 mL) to dry 1,2-dichloroethane, 1-O-acetyl-2,3,5-tri-O-benzoyl-β-d-ribofuranose 5 (2.1 g, 0.004 mol), and trimethylsilyltrifluoromethanesulfonate (6 mL) was used as a catalyst. After the solution had been stirred for 3 weeks (TLC) at room temperature, it was washed with a saturated solution of aqueous sodium bicarbonate (3 × 20 mL) and water (3 × 20 mL), and dried over anhydrous sodium sulfate. The pure product was separated using silica gel column chromatography with chloroform and ester (90:2), which produced a yellow solid. Yield 0.1083 g, (2.88%); m.p. 110 °C; 1HNMR CDCl3: 8.08–7.26 (m, 1H) Aromatic protons; 6.69 (d, 1H, J = 5.1 Hz) H1’’, 5.90(t, 1H)H2’’, 5.80–5.78 (q, 1H) N-CH-N; 5.69–5–63 (ds,1H; J = 4.25 Hz) H1’; 5.59–5.47 (tt, 1H )H2’; 5.35 (d, 1H, J = Hz) H3’’; 5.15–5.09 (d,1H, J = 5.1 Hz) H3’; 4.80–4.60 (m, 1H) H4’’; 4.59–4.47 (dd,1H; J = 5.1 Hz) H4’; 4.49–4.35 (m,1H) H5’’; 3.81–3.45 (m,1H) H5’; sugars protons; 1.25–1.4 (dm, 3H, 7JH–F = 6.8 Hz) CH3; 13CNMR CDCl3: 166.50, (166.20d, JC–F = 12.78 Hz), 166.07, (165.60d, JC–F = 23.43), 165.47, 165.36, 165.24 C=O groups, 133.67, 133.59, 133.53, 133.46, 133.36, 133.24, 133.18, 133.12, 129.88, 129.84, 129.79, 129.76, 129.65, 129.50, 129.19, 129.04, 128.92, 128.84, 128.63, 128.58, 128.51, 128.45, 128.41, 128.35, 128.47, 128.37, 127.28, 107.35, 104.87, 100.49, 95.85, 80.85, (79.68d C2’ JC-F = 14.91 Hz), 78.33, 76.15, (74.91d, JC-F = 72.42 Hz), 74.57, 72.33, (71.90dt JC–F = 31.95C3’), (65.17d, JC–F = 6.39 Hz), 64.74, 64.16, 63.73 N-CH-N, 22.71 CH3. Formula C62H49F3N2O15; M.wt: 1119.05.
- Deprotection of 1,3-bis-(2,3,5-tri-O-Benzoyl-β-d-Ribofuranosyl)-8-(Trifluoromethyl)-2-Methyl-4-Quinazolinone: Synthesis of 2-Methyl-1,3-bis-(β-d-Ribofuranosyl)-8-(Trifluoromethyl)-4-Quinazolinone 7. The protected nucleoside (0.2mmol) 6, absolute methanol (20 mL) and sodium metal (0.013 g, 0.5 mmol) was stirred at room temperature for 24 h (TLC). The solvent was evaporated under vacuum and the residue was dissolved in hot water and neutralized with few drops of acetic acid. The precipitate formed was filtered, dried, and crystallized from water to leave yellow crystals of free nucleoside 7.
4. Results and Discussion
5. Conclusions
Acknowledgments
References
- Asif, M. Chemical Characteristics, Synthetic Methods, and Biological Potential of Quinazoline and Quinazolinone Derivative’. Int. J. Med. Chem. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Alafeefy, A.M. Some new quinazolin-4(3H)-one derivatives, synthesis and antitumor activity. J. Saudi Chem. Soc. 2011, 15, 337–343. [Google Scholar] [CrossRef]
- Abbas, S.E.; Barsoum, F.F.; Georgey, H.H.; Mohammed, E.R. Synthesis and antitumor activity of certain 2,3,6-trisubstituted quinazolin-4(3H)-one derivatives. In Bulletin of Faculty of Pharmacy; Cairo University: Giza, Egypt, 2013; Volume 51, pp. 273–282. [Google Scholar] [CrossRef]
- Break, L.M. Synthesis and Characterization of New 8-trifluloromethyl Quinazolin-2,4-(3H)-Dione Nucleosides. Int. J. Chem. 2017, 9. [Google Scholar] [CrossRef]
- Break, L.M.; Mohamed, M.; Abdel-Hafez, S.H. Synthesis of New Organoselenium Compounds Containing Nucleosides as Antioxidant. Orient. J. Chem. 2014, 30, 1639–1645. [Google Scholar] [CrossRef]
- Break, L.M.; Mosselhi, M.A.; Elshafai, N.M. Nucleosides 8 [18]: Ribosylation of Fused Quinazolines—Synthesis of New [1,2,4]Triazolo[5,1-b]- and [1,2,4]Triazino[3,2-b]quinazoline Nucleosides of Fluorescence Interest. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Break, L.M.; Mosselhi, A.N.M. Synthesis, structure and Antimicrobial activity of new 3- and 2-arylmethyl and arylacyl-3H [1,2,4] triazino [3,2-b]-quinazoline-2,6 (1H) diones as expect as DNA fluorphores. Res. J. Chem. Sci. 2012, 2, 23–28. [Google Scholar]
- Break, L.M. Synthesis of Some of Fluorinated Benzimidazole Nucleosides. Int. J. Chem. 2016, 8, 188. [Google Scholar] [CrossRef]
- Break, L.M. Synthesis of the Novel 3-Benzotriazole-5-yl difluoromethyl-5-trifluoromethyl benzotriazole Nucleosides. Int. J. Chem. 2015, 7, 99. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wahshi, F.S.; Alqahtani, M.D.; Abdulla, M.; Al-Hemyari, A.; Bufaroosha, M.; Ramachandran, T.; Hamed, F.; Thiemann, T. Our Experience of Using Thermally Recycled Silica Gel in a Teaching and Small Research Laboratory Setting. Proceedings 2019, 9, 28. https://doi.org/10.3390/ecsoc-22-05696
Wahshi FS, Alqahtani MD, Abdulla M, Al-Hemyari A, Bufaroosha M, Ramachandran T, Hamed F, Thiemann T. Our Experience of Using Thermally Recycled Silica Gel in a Teaching and Small Research Laboratory Setting. Proceedings. 2019; 9(1):28. https://doi.org/10.3390/ecsoc-22-05696
Chicago/Turabian StyleWahshi, Fatima Sbait, Maitha Dhaiman Alqahtani, Manhal Abdulla, Abdullah Al-Hemyari, Muna Bufaroosha, Tholkappiyan Ramachandran, Fathalla Hamed, and Thies Thiemann. 2019. "Our Experience of Using Thermally Recycled Silica Gel in a Teaching and Small Research Laboratory Setting" Proceedings 9, no. 1: 28. https://doi.org/10.3390/ecsoc-22-05696
APA StyleWahshi, F. S., Alqahtani, M. D., Abdulla, M., Al-Hemyari, A., Bufaroosha, M., Ramachandran, T., Hamed, F., & Thiemann, T. (2019). Our Experience of Using Thermally Recycled Silica Gel in a Teaching and Small Research Laboratory Setting. Proceedings, 9(1), 28. https://doi.org/10.3390/ecsoc-22-05696




