BODIPY Derivatives: Synthesis and Evaluation of Their Optical Properties †
Abstract
:1. Introduction
2. Experimental Section
2.1. Methods and Materials
2.2. Synthesis of BODIPY Derivatives
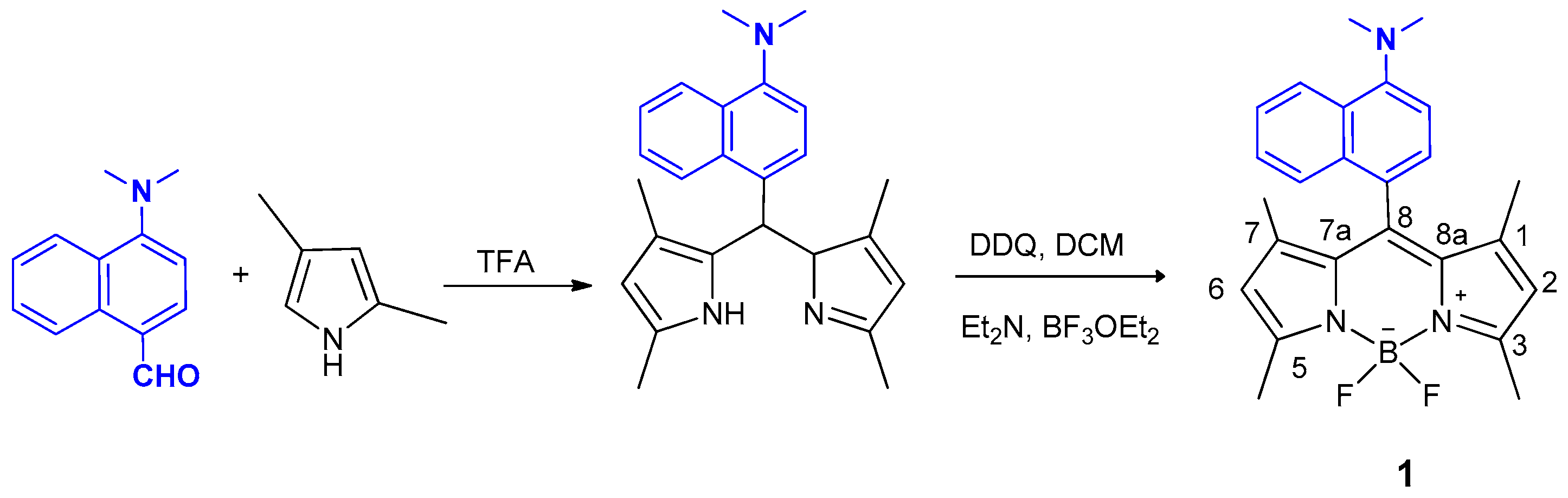
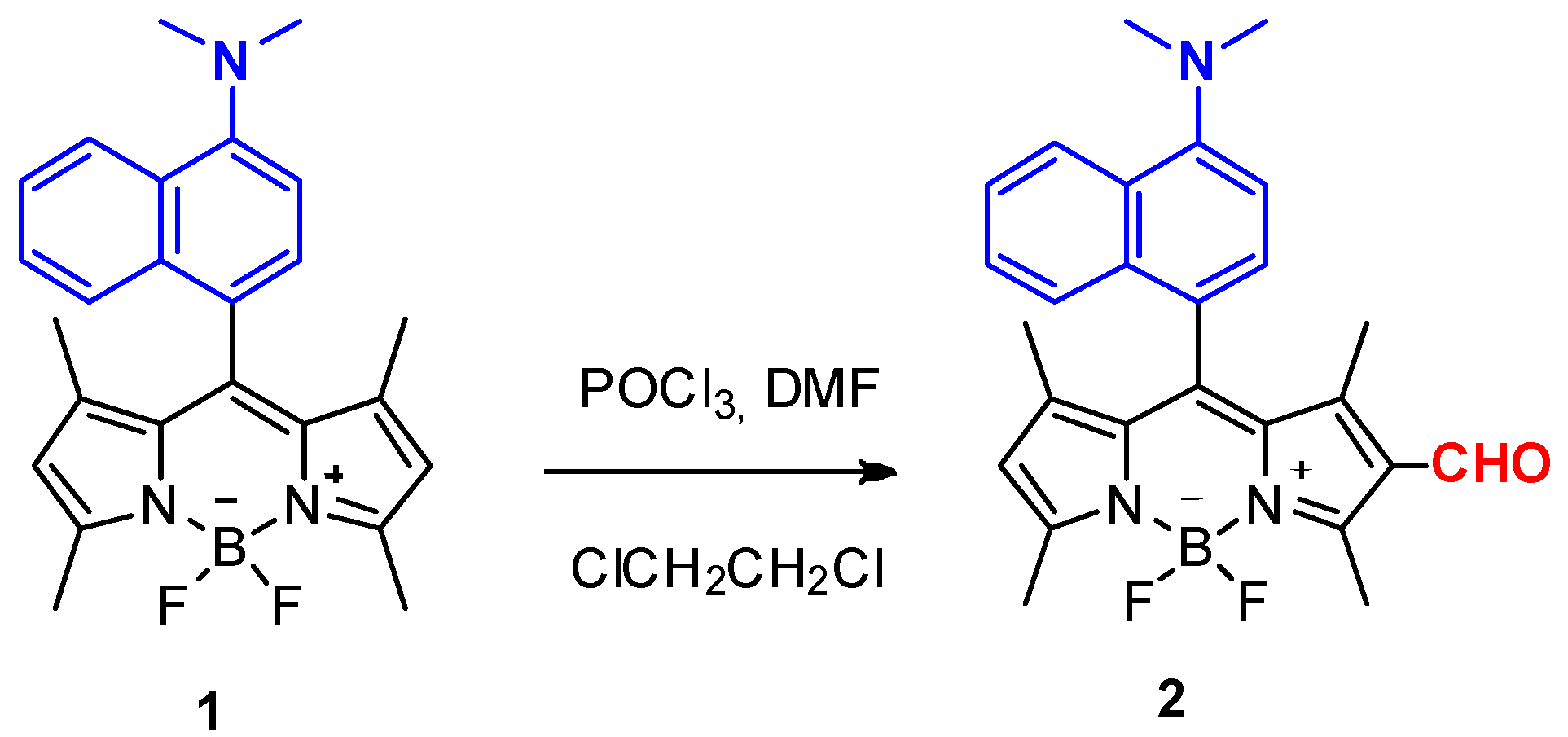
2.2.1. BODIPY Derivative 1
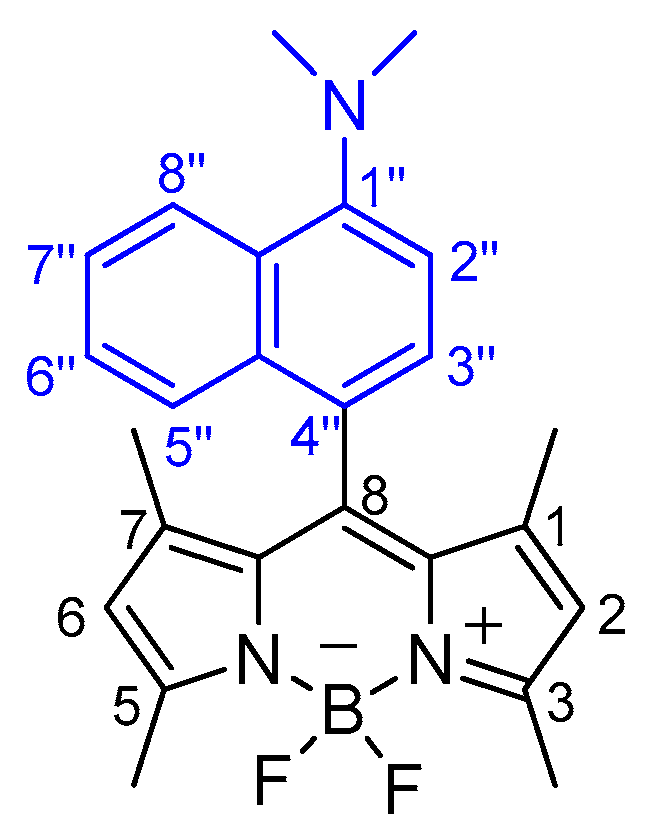
2.2.2. BODIPY Derivative 2
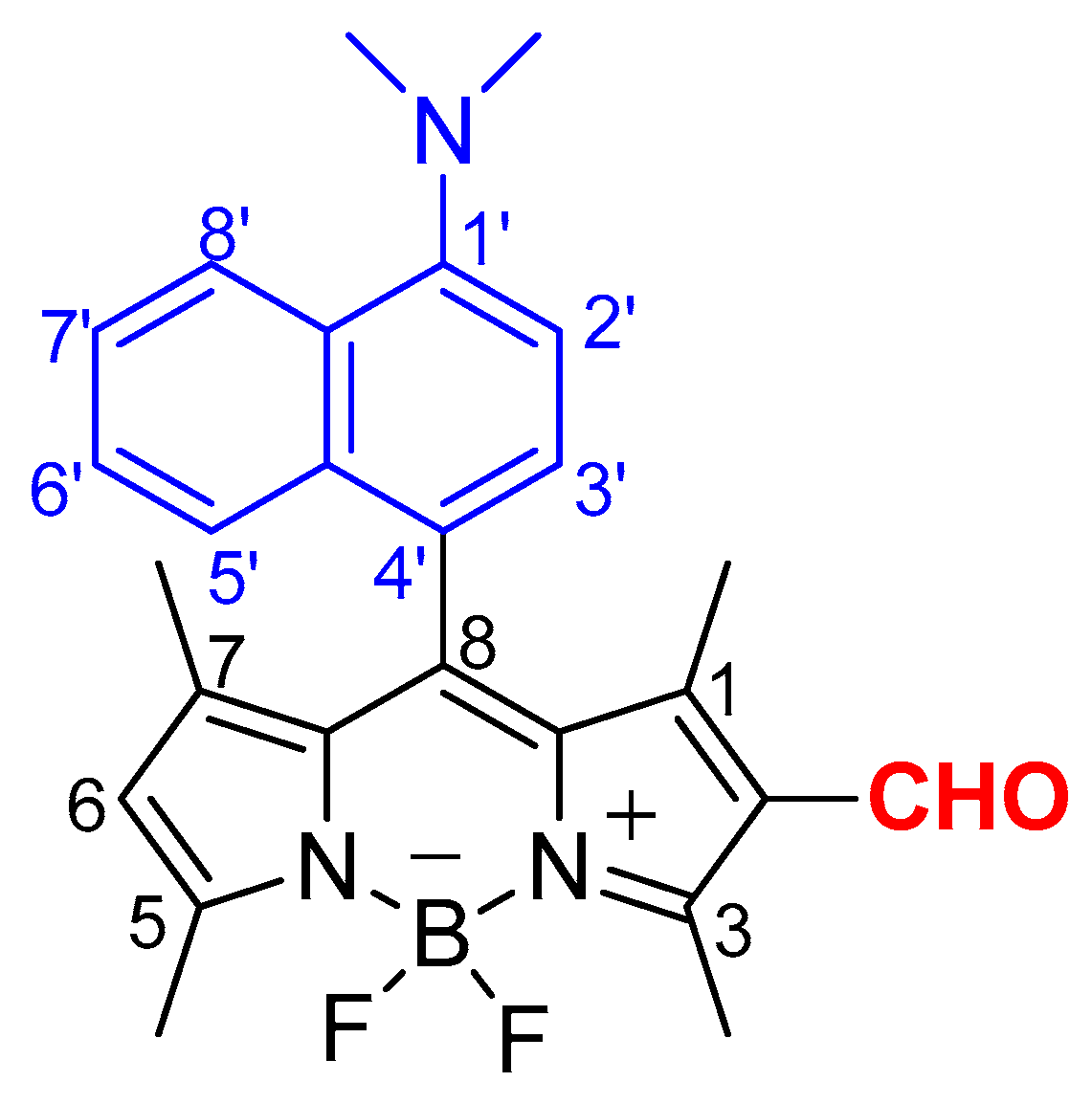
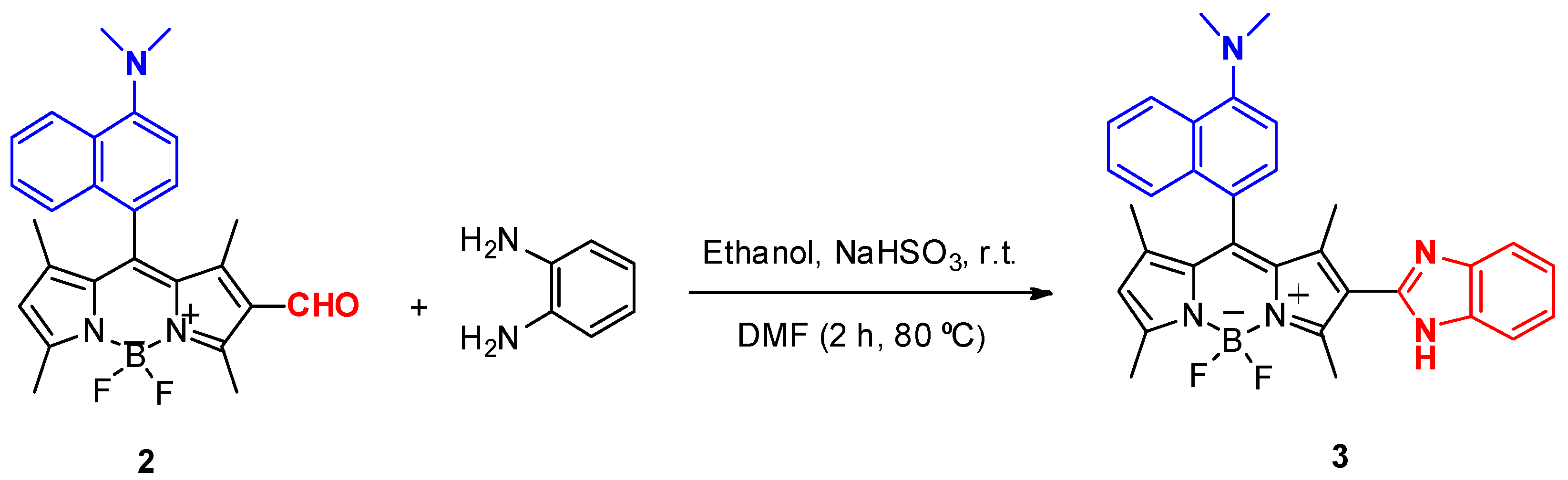
2.2.3. BODIPY Derivative 3
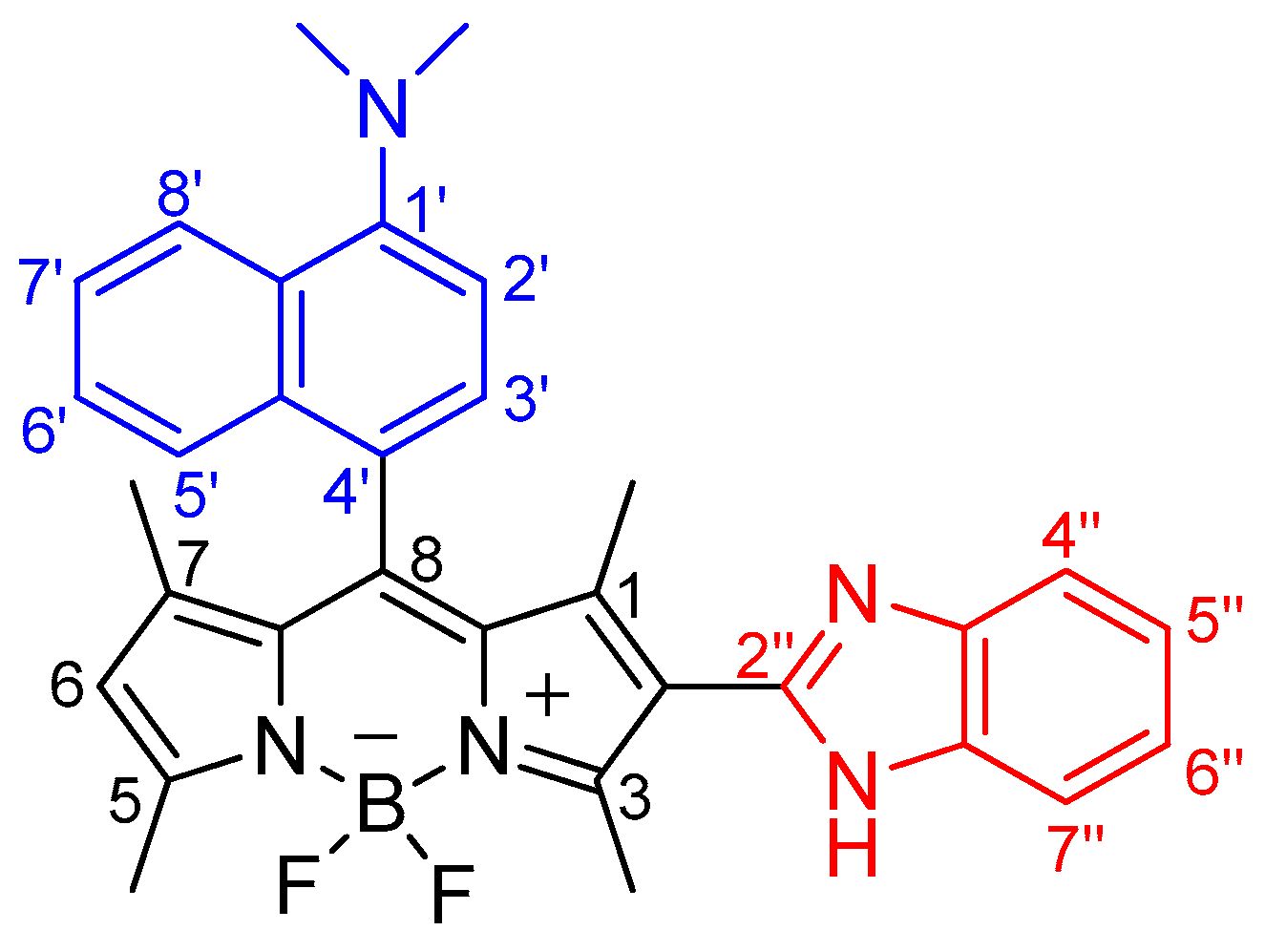
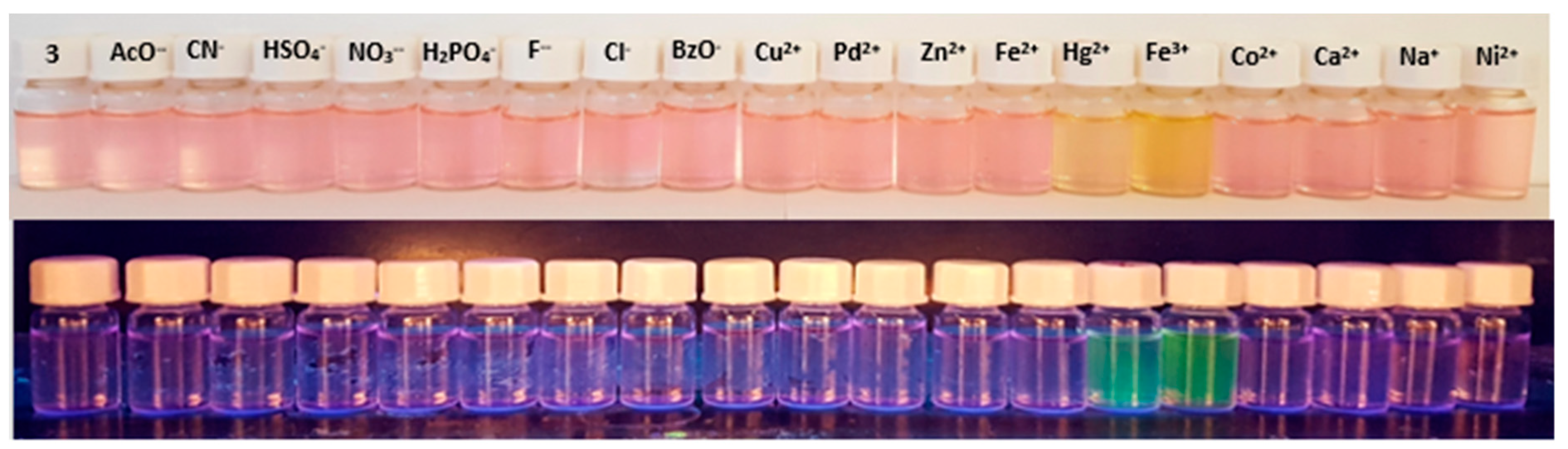
2.3. Study on the Sensing Ability of BODIPY Derivative 3
3. Results and Discussion
3.1. Synthesis of BODIPY Derivatives
3.2. Photophysical Characterization of BODIPY Derivatives
3.3. Preliminary Study on the Sensing Ability of BODIPY Derivative 3
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef] [PubMed]
- Loudet, A.; Burgess, K. BODIPY dyes and their derivatives: Syntheses and spectroscopic properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef]
- Freidus, L.G.; Pradeep, P.; Kumar, P.; Choonara, Y.E.; Pillay, V. Alternative fluorophores designed for advanced molecular imaging. Drug Discov. Today 2018, 23, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Presti, M.L.; Martínez-Máñez, R.; Ros-Lis, J.V.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M. , Sancenón, F. A dual channel sulphur-containing macrocycle functionalised BODIPY probe for the detection of Hg(II) in mixed aqueous solution. New J. Chem. 2018, 42, 7863–7868. [Google Scholar] [CrossRef]
- Collado, D.; Casado, J.; González, S.R.; Navarrete, J.T.L.; Suau, R.; Perez-Inestrosa, E.; Pappenfus, T.M.; Raposo, M.M.M. Enhanced functionality for donor-acceptor oligothiophenes via inclusion of BODIPY: Synthesis, electrochemistry, photophysics and model chemistry. Chem. Eur. J. 2011, 17, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Demas, J.N.; Crosby, G.A. Measurement of photoluminescence quantum yields. Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar]

| BODIPY Derivatives | UV–vis | Fluorescence | |||
|---|---|---|---|---|---|
| λmax (nm) | log ε | λem (nm) | ΦF | Stokes’ Shift (nm) | |
| 1 | 500 | 4.58 | 512 | 0.117 | 12 |
| 2 | 494 | 4.75 | 512 | 0.148 | 15 |
| 3 | 512 | 4.75 | 514 | 0.031 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, R.C.R.; Nogueira, M.B.; Costa, S.P.G.; Raposo, M.M.M. BODIPY Derivatives: Synthesis and Evaluation of Their Optical Properties. Proceedings 2019, 9, 10. https://doi.org/10.3390/ecsoc-22-05700
Gonçalves RCR, Nogueira MB, Costa SPG, Raposo MMM. BODIPY Derivatives: Synthesis and Evaluation of Their Optical Properties. Proceedings. 2019; 9(1):10. https://doi.org/10.3390/ecsoc-22-05700
Chicago/Turabian StyleGonçalves, Raquel C. R., Mariana B. Nogueira, Susana P. G. Costa, and M. Manuela M. Raposo. 2019. "BODIPY Derivatives: Synthesis and Evaluation of Their Optical Properties" Proceedings 9, no. 1: 10. https://doi.org/10.3390/ecsoc-22-05700
APA StyleGonçalves, R. C. R., Nogueira, M. B., Costa, S. P. G., & Raposo, M. M. M. (2019). BODIPY Derivatives: Synthesis and Evaluation of Their Optical Properties. Proceedings, 9(1), 10. https://doi.org/10.3390/ecsoc-22-05700






