Comparison of Untapped Agroindustrial Olive Resources with Olive Leaves †
Abstract
:1. Introduction
2. Material and Methods
2.1. Olive-Derived Biomasses and Chemical Characterization
2.2. Extraction Method
2.3. Measurement of the Total Phenol Content and Antioxidant Capacity
2.4. Reversed-Phase High Performance Liquid Chromatography Analyses
3. Results and Discussion
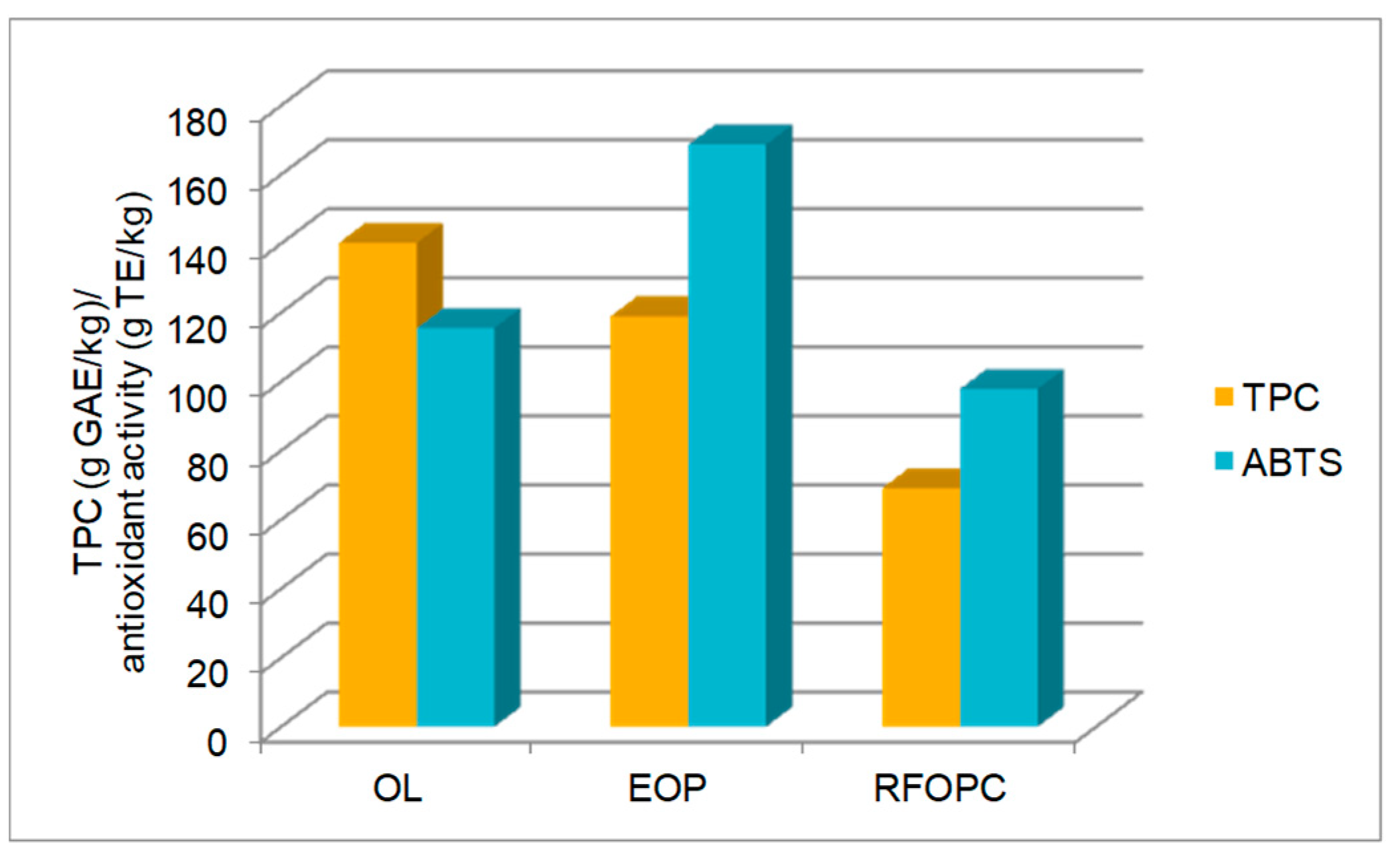
3.1. Chemical Composition and Antioxidant Characteristics
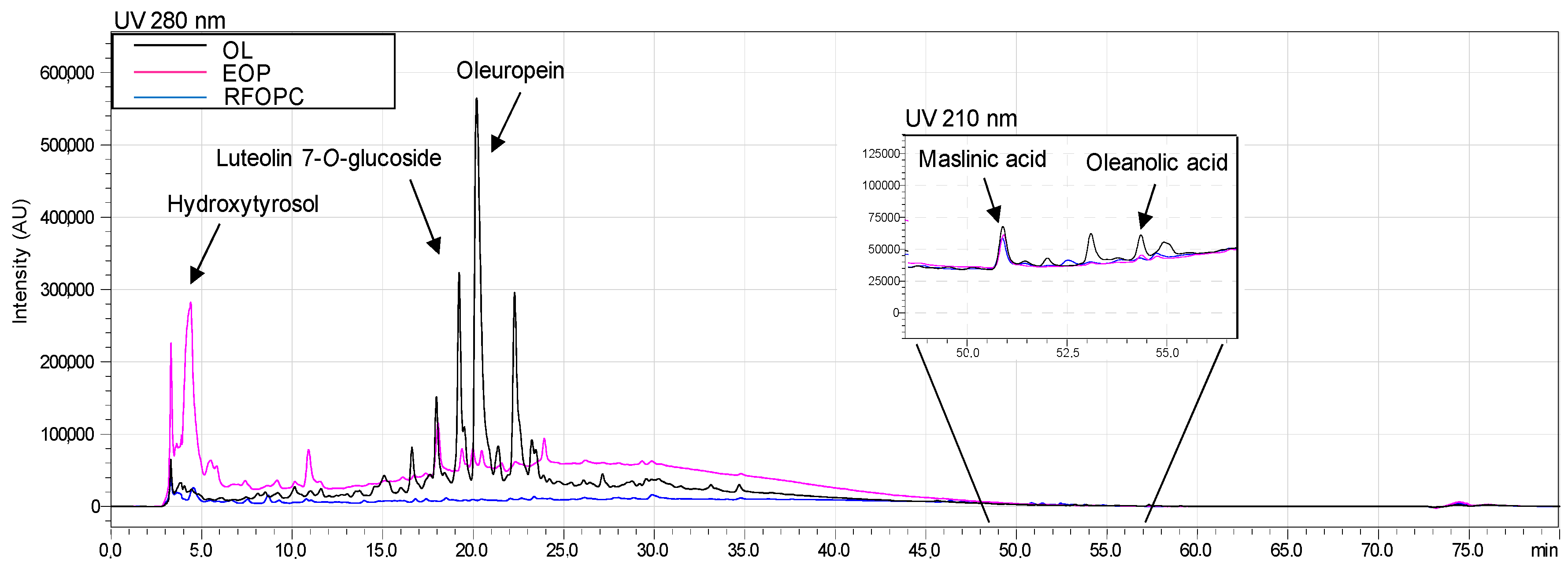
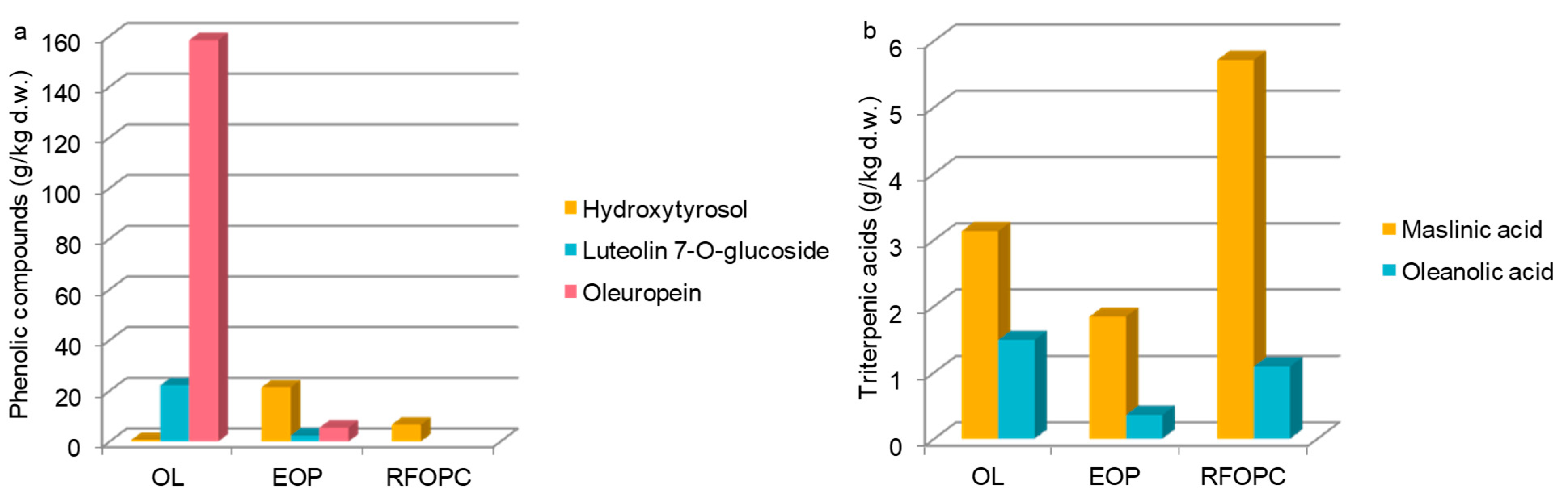
3.2. HPLC Profiles and Quantitative Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De La Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Determination of phenolic compounds of ‘Sikitita’ olive leaves by HPLC-DAD-TOF-MS. Comparison with its parents ‘Arbequina’ and ‘Picual’ olive leaves. LWT-Food Sci. Technol. 2014, 58, 28–3 4. [Google Scholar] [CrossRef]
- Talhaoui, N.; Gómez-Caravaca, A.M.; León, L.; De La Rosa, R.; Fernández-Gutiérrez, A.; Segura-Carretero, A. From olive fruits to olive oil: Phenolic compound transfer in six different olive cultivars grown under the same agronomical condition. Int. J. Mol. Sci. 2016, 17, 337. [Google Scholar] [CrossRef] [PubMed]
- Contreras, M.d.M.; Romero, I.; Moya, M.; Castro, E. Olive-derived biomass as a renewable source of value-added products. Process Biochem. 2020, 97, 43–56. [Google Scholar] [CrossRef]
- Talhaoui, N.; Taamalli, A.; Gómez-Caravaca, A.M.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015, 77, 92–108. [Google Scholar] [CrossRef]
- Lopez-Huertas, E.; Fonolla, J. Hydroxytyrosol supplementation increases vitamin C levels in vivo. A human volunteer trial. Redox Biol. 2017, 11, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef]
- Echeverría, F.; Ortiz, M.; Valenzuela, R.; Videla, L.A. Hydroxytyrosol and cytoprotection: A Projection for Clinical Interventions. Int. J. Mol. Sci. 2017, 18, 930. [Google Scholar] [CrossRef]
- Medfai, W.; Contreras, M.d.M.; Lama-Muñoz, A.; Mhamdi, R.; Oueslati, I.; Castro, E. How cultivar and extraction conditions affect antioxidants type and extractability for olive leaves valorization, ACS Sustain. Chem. Eng. 2020, 8, 5107–5118. [Google Scholar]
- Contreras, M.d.M.; Lama-Muñoz, A.; Gutiérrez-Pérez, J.M.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Integrated process for sequential extraction of bioactive phenolic compounds and proteins from mill and field olive leaves and effects on the lignocellulosic profile. Foods 2019, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Ali Mami, M.; Mätzing, H.; Gehrmann, H.J.; Stapf, D.; Bolduan, R.; Lajili, M. Investigation of the olive mill solid wastes pellets combustion in a counter-current fixed bed reactor. Energies 2018, 11, 1965. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Laboratory Analytical Procedure TP-510-42623: Determination of sugars, byproducts, and degradation products in liquid fraction process samples. Natl. Renew. Energy Lab. 2006, 1–14. [Google Scholar]
- Contreras, M.d.M.; Lama-Muñoz, A.; Espínola, F.; Moya, M.; Romero, I.; Castro, E. Valorization of olive mill leaves through ultrasound-assisted extraction. Food Chem. 2020, 314, 126218. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; Cara, C.; Romero, I.; Castro, E.; Gullón, B. Valorisation of exhausted olive pomace by an eco-friendly solvent extraction process of natural antioxidants. Antioxidants 2020, 9, 1010. [Google Scholar] [CrossRef]
- Ammar, S.; Contreras, M.d.M.; Gargouri, B.; Segura-Carretero, A.; Bouaziz, M. RP-HPLC-DAD-ESI-QTOF-MS based metabolic profiling of the potential Olea europaea by-product “wood” and its comparison with leaf counterpart. Phytochem. Anal. 2017, 28, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Navarro, M.; Morales, F.J.; Ramos, S. Olive leaf extract concentrated in hydroxytyrosol attenuates protein carbonylation and the formation of advanced glycation end products in a hepatic cell line (HepG2). Food Funct. 2017, 8, 944–953. [Google Scholar] [CrossRef]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative study on beneficial effects of hydroxytyrosol- and oleuropein-rich olive leaf extracts on high-fat diet-induced lipid metabolism disturbance and liver injury in rats. BioMed Res. Int. 2020, 2020. [Google Scholar] [CrossRef] [PubMed]
- Ning, S.; Dongli, L.; Xiaoqing, C.; Panpan, W.; Yu-Jing, L.; Ning, H.; Wen-Hua, C.; Wing-Leung, W. New applications of oleanolic acid and its derivatives as cardioprotective agents: A review of their therapeutic perspectives. Curr. Pharm. Des. 2019, 25, 3740–3750. [Google Scholar]
- Xie, P.; Huang, L.; Zhang, C.; Deng, Y.; Wang, X.; Cheng, J. Enhanced extraction of hydroxytyrosol, maslinic acid and oleanolic acid from olive pomace: Process parameters, kinetics and thermodynamics, and greenness assessment. Food Chem. 2019, 276, 662–674. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contreras, M.d.M.; Gómez-Cruz, I.; Romero, I.; Castro, E. Comparison of Untapped Agroindustrial Olive Resources with Olive Leaves. Proceedings 2021, 79, 3. https://doi.org/10.3390/IECBM2020-08585
Contreras MdM, Gómez-Cruz I, Romero I, Castro E. Comparison of Untapped Agroindustrial Olive Resources with Olive Leaves. Proceedings. 2021; 79(1):3. https://doi.org/10.3390/IECBM2020-08585
Chicago/Turabian StyleContreras, María del Mar, Irene Gómez-Cruz, Inmaculada Romero, and Eulogio Castro. 2021. "Comparison of Untapped Agroindustrial Olive Resources with Olive Leaves" Proceedings 79, no. 1: 3. https://doi.org/10.3390/IECBM2020-08585
APA StyleContreras, M. d. M., Gómez-Cruz, I., Romero, I., & Castro, E. (2021). Comparison of Untapped Agroindustrial Olive Resources with Olive Leaves. Proceedings, 79(1), 3. https://doi.org/10.3390/IECBM2020-08585






