Abstract
Osteosarcoma (OS) is a rare, aggressive bone tumor that impacts mostly children and young adults. Despite numerous therapeutic efforts, OS still presents a low patient survival rate, high metastasis, and relapse occurrence. To surpass that, polymeric micelles have been researched for the targeted co-delivery of genetic material and drugs. In this work, mixed polymeric micelles with cationic properties containing polyethyleneimine (PEI), Pluronics® F68 and P123 were prepared. Pluronic® F68 was activated by addition of diacrylate groups and conjugated with PEI. Pluronic® P123 was incorporated in the formulation in a ratio of 2:1 regarding the concentration of Pluronic® F68-PEI and Pluronic® P123. The nanosystems were structurally characterized by FTIR and NMR spectroscopy and the morphology was assessed by TEM. Particle size, polydispersity index (PDI) and zeta potential were assessed by Dynamic and Electrophoretic Light Scattering, respectively. Small-sized, irregularly shaped F68-PEI micelles were obtained, with a PDI of 0.346 and zeta potential of 12.59 mV. Incorporating Pluronic® P123 in the formulation lowered particle size and resulted in spherical micelles. Zeta potential decreased due the presence of Pluronic® P123, but remained positive. These results indicate a stable, small-sized nanosystem, characteristics that suggest a capability to surpass multidrug resistance and perform active targeting towards OS.
1. Introduction
Osteosarcoma (OS) is a rare bone tumor with an incidence of about 4 per million people annually. Despite this, it is one of the most common cancers affecting adolescents, behind lymphoma and brain tumors [1]. Throughout the past few decades, little to no advancement regarding the prognosis of this disease has been achieved, amidst numerous research efforts, and children and young adults present a worse prognostic [2].
The panorama of OS is characterized by a low patient survival rate that has not improved in the last decades, particularly in patients suffering from metastatic tumors or presenting a local tumor in an advanced stage by the time of diagnosis. Moreover, regarding the patients that experience disease relapse, treatment will depend on whether tumor resection is possible, on the prior chemotherapy regimen and the time frame until tumor relapse [2].
OS treatment has been met with several limitations over the years that have both impaired patient safety and stalled tumor eradication. The most common feature in patients suffering from metastatic OS is resistance to chemotherapy, as it is responsible for disease recurrence after a disease-free period, typically with a more aggressive tumor form or metastatic type, which contributes to treatment failure [3,4]. As it is presumed that significant alterations in the current chemotherapy scenario will not result in an improved OS prognosis, there has been an increasing effort in the discovery of new therapeutic alternatives [5]. Moreover, the evident lack of therapeutic agents capable of performing specific antitumor activity on the tumor site limits their efficiency, as the drug’s blood distribution is affected by several physiological responses or barriers, and the therapeutic dosage administered is often lowered to avoid considerable cytotoxicity to normal cells [6]. Since these problems, and others, including poor drug pharmacokinetics, high cellular toxicity and drug resistance are still present in the panorama of OS, several nanomedicine-based studies have been conducted, seeking to overcome these challenges [7].
The term nanomedicine refers to the use of nano-sized materials for therapeutic and imaging purposes, useful for the diagnosis, monitoring and treatment of tumors. Currently, several nano-delivery systems are being researched for cancer treatment, such as liposomes, metallic nanoparticles, solid lipid nanoparticles, dendrimers, albumin nanoparticles and polymeric micelles (PM) [8,9]. PMs are core-shell nanostructures, usually 10–200 nm, composed of two chemically diverse blocks, namely a hydrophilic and a hydrophobic segment [10,11].
As drug delivery systems, PMs present some advantageous characteristics. Their small size allows for tissue penetration and passive tumor targeting, and nano-sized particles are also able to avoid the reticuloendothelial system and recognition by macrophages, resulting in a higher blood circulation time [10,12]. As their shape and size can be controlled, these systems achieve a higher drug load capacity [13]. Because PMs can improve the solubility of chemotherapeutic agents in water, therefore leading to an increased systemic half-life, PM-bound drugs benefit from the enhanced permeation and retention (EPR) effect. This EPR effect allows the passive accumulation of the drug in the tumor locally due to the abnormal and high density of tumor vasculature [14]. Additionally, the outer hydrophilic block can be tailored by adding ligands to further improve the efficiency and active targeting of a PM [10,11].
Poloxamers, or Pluronics®, are non-ionic surfactants commonly used in PMs, structurally composed of A-B-A triblock copolymers, with A being poly(ethylene oxide) (PEO) and B representing poly(propylene oxide) (PPO) [15]. They possess an amphiphilic nature, with hydrophobic PPO blocks and hydrophilic PEO blocks [16].
Cationic polymers are frequently conjugated with PMs, as they aid the transfection of nucleic acids by the establishment of electrostatic interactions, which, in turn, facilitates their cellular uptake and escape from enzymatic degradation, allowing for gene therapy. Polyethyleneimine (PEI) is the most frequently used synthetic cationic polymer, possessing primary, secondary and tertiary amino groups. As previously mentioned, when conjugated with nucleic acids, PEI can spontaneously establish electrostatic interactions between its protonable amino groups and the phosphate groups in the nucleic acids, forming polyelectrolyte complexes [17,18]. This polymer has also shown high transfection efficiency in in vitro and in vivo testing. It also presents an ability to escape from the endosome, by a mechanism called “proton sponge”, releasing the genetic material in the cell [19].
The aim of this work consisted of the development, optimization and structural and morphological characterization of a PM containing Pluronic® F68 complexed with PEI and, alternatively, the addition of Pluronic® P123, in order to determine the best formulation to achieve a stable, small-sized micelle capable of active targeting to OS cells and to surpass multiple drug resistance.
2. Experiments
2.1. Materials
Pluronic® F68 (MW: 8400 Da), Pluronic® P123 (MW: 5800 Da), anhydrous toluene, hexane and anhydrous dichloromethane were purchased from Sigma-Aldrich (St. Louis, MO, USA). Branched PEI 99% (MW: 1800 Da) and acryloyl chloride were acquired from Alfa Aesar (Haverhill, MA, USA). Triethylamine was purchased from Merck (Darmstadt, Germany). Regenerated cellulose dialysis tubing (MWCO = 6000–8000 Da) was acquired from Orange Scientific (Braine l’Alleud, Belgium). Cellulose acetate syringe filters were purchased from Filter-Lab® (Barcelona, Spain).
2.2. Methods
2.2.1. Synthesis of Pluronic® F68 Diacrylate
The synthesis of Pluronic® F68 diacrylate was carried out as previously described [20]. 7.76 g of Pluronic® F68 was weighed in an Erlenmeyer flask and dissolved in 80 mL of anhydrous toluene. The solution was transferred to a three-neck round bottom flask. Next, 1.5 mL of triethylamine and 0.75 mL of acryloyl chloride (diluted in 15 mL of toluene) were added to the solution, dropwise. The reaction was heated at a temperature of 70 °C for 6 h and then stirred at room temperature overnight under inert atmosphere (dry nitrogen). After that, the reaction was subjected to a filtration step to remove triethylamine-hydrochloride and toluene was evaporated on a rotary evaporator. The next step involved the addition of 3 × 15 mL of ice-cold anhydrous hexane to the Erlenmeyer flask containing raw Pluronic® F68 diacrylate under continuous stirring, which yielded a yellowish precipitate. Finally, Pluronic® F68 diacrylate was separated from the hexane through filtration and dried in a vacuum oven at room temperature (25 °C) overnight under reduced pressure and stored in a desiccator until further use. 6.42 g (81.4%) of product was obtained.
2.2.2. Synthesis of Pluronic® F68 Diacrylate Complexed with PEI
For the synthesis of Pluronic® F68 complexed with the cationic polymer PEI, 0.404 g of Pluronic® F68 diacrylate was weighed into an Erlenmeyer and dissolved in 10 mL of anhydrous dichloromethane. Likewise, 0.460 g of PEI was weighted in a round bottom flask and dissolved in 20 mL of dichloromethane. Next, the diacrylate solution was added dropwise, under stirring, to the PEI solution, and the round bottom flask was stirred for 48 h at 40 °C. After this, the flask was placed in a rotary evaporator at 40 °C until the solvent evaporated. The reaction was then transferred to a vial and stored in a desiccator.
Furthermore, the dry product was dissolved with the help of a sonic bath, in 20 mL of deionized water (10 mL per 0.5 g), and was subsequently subjected to a dialysis step, using a membrane with a molecular weight cut-off of 6000–8000 Da, during 48 h at 4 °C, against distilled water, in order to remove the unconjugated products. The dialysis process was monitored by observing the absorbance at around 210 nm by UV spectroscopy. Finally, the dialyzed solution was moved to a 50 mL falcon, frozen at −80 °C and lyophilized. The lyophilized product was stored in a desiccator.
2.2.3. Synthesis of Pluronic® F68 Diacrylate Complexed with PEI and Pluronic® P123
Mixed micelles with Pluronic® P123 were achieved by adding the poloxamer to the lyophilized Pluronic® F68/PEI reaction. Stock solutions of Pluronic®F68-PEI and Pluronic® P123 were prepared by weighing 10 mg of each compound and dissolving them in 1 mL of deionized water. Different ratios of prepared stock solutions were tested (1:1, 1:2 and 2:1) at a total concentration of 1 mg/mL.
3. Results
3.1. Synthesis of Pluronic® F68 Diacrylate
The formation of Pluronic F68 diacrylate was performed by end-capping acryloyl groups to the hydroxyl groups of Pluronic F68. Briefly, acryloyl chloride was used as the activating agent, under triethylamine (Et3N) used as a base. The formation of the reaction product was confirmed by 1H-NMR and FTIR spectroscopy.
The 1H-NMR spectra (Figure 1), that compares Pluronic F68 and Pluronic F68 diacrylate, shows the appearance of new peaks (identified with the number ‘1’) in the spectrum of Pluronic F68 diacrylate due to the olefinic diacrylate protons in the range of δ5.8–6.4 ppm, and a peak shift from δ4.5 ppm in the spectrum for the unadulterated Pluronic® to δ4.3 ppm in the Pluronic F68 diacrylate spectrum.
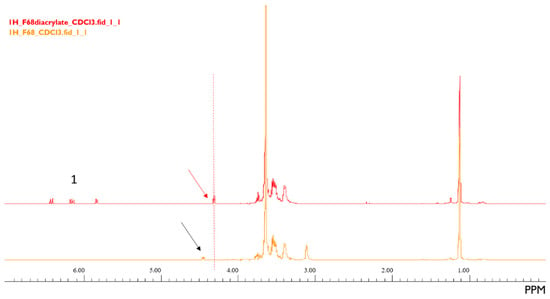
Figure 1.
1H-NMR spectra of Pluronic® F68 (orange) and Pluronic® F68 diacrylate (red). The peaks identified with the number ‘1’ were formed after the activation of Pluronic F68. A slight peak shift was observed.
In the FTIR spectroscopy (Figure 2), a new band in the 1725 cm−1 region of the F68 diacrylate spectrum appeared due to the C=O bond vibration.
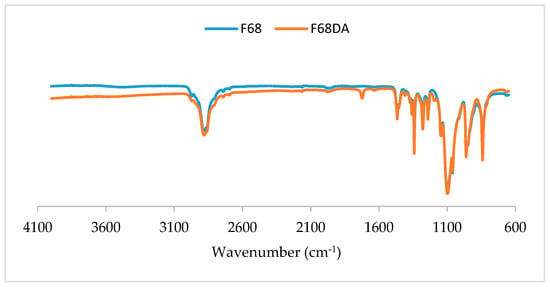
Figure 2.
FTIR spectra of Pluronic® F68 and Pluronic® F68 diacrylate. A new band is identified in the spectrum for Pluronic F68 diacrylate, in the 1725 cm−1 region.
3.2. Synthesis of Pluronic® F68 Diacrylate Complexed with PEI
PEI was dissolved in anhydrous dichloromethane for 48 h at 40 °C. The product was dialyzed and the properties of F68 grafted with PEI were measured by Dynamic Light Scattering (DLS) for its particle size, polydispersity index (PDI) and zeta potential values after the lyophilization process (Table 1). An accentuated reduction in the particle size for the complex formed with PEI following filtration with a 0.22 µm cellulose acetate filter is notable, from 546.6 to 153.3 nm. The PDI also decreased, from 0.549 to 0.346.

Table 1.
Characteristics of the complex formed between Pluronic® F68 diacrylate with polyethyleneimine (PEI) in dichloromethane following the lyophilization process, before and after filtration.
The success of F68 grafting was confirmed by 1H-NMR and FTIR spectroscopy. The 1H-NMR spectra are presented in Figure 3. In the PEI spectrum, a peak can be identified around δ2.6 ppm. For the F68/PEI spectrum, peaks around the same ppm zone are observed, which do not appear in the spectrum for the diacrylate.
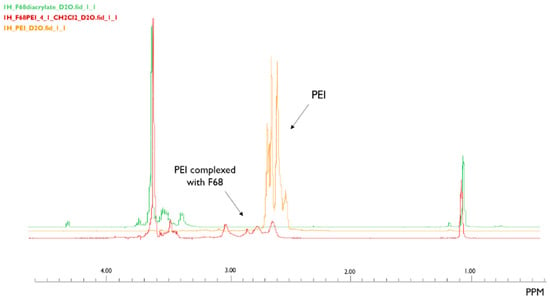
Figure 3.
1H-NMR spectra of PEI (orange), Pluronic® F68 diacrylate complexed with PEI (red), and Pluronic® F68 diacrylate (green). Peaks in the F68/PEI spectrum are coincident with the peak observed in the PEI spectrum. The same peak is not visible in F68 diacrylate.
The FTIR spectra for Pluronic® F68 diacrylate and F68/PEI showed a band appearing in the latter spectrum, around 3400 cm−1, due to the amine N–H vibrations as indicated in Figure 4.
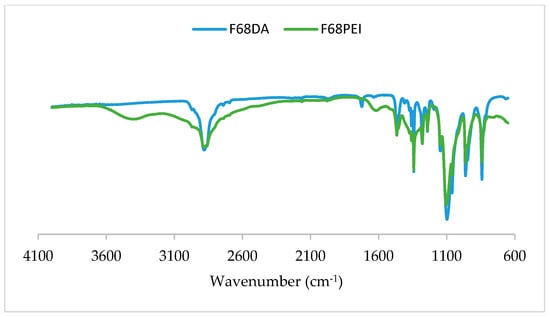
Figure 4.
FTIR spectra of Pluronic® F68 diacrylate and Pluronic® F68 diacrylate complexed with PEI. The PEI band is identified at 3400 cm−1 (amine N–H vibrations).
3.3. Synthesis of Pluronic® F68 Diacrylate Complexed with PEI and Pluronic® P123
Three ratios (%v/v) of Pluronic® F68/PEI to Pluronic® P123 were tested, namely 1:1, 1:2 and 2:1. Stock solutions of Pluronic® F68/PEI and Pluronic® P123 were prepared by weighing 10 mg of each compound and diluting them in 1 mL of deionized water, and the ratios were prepared in order to obtain 1 mL of solution with the total concentration of 1 mg/mL. DLS measurements were then performed. The results in Table 2 show the values following filtration.

Table 2.
Characteristics of the complexes formed between Pluronic® F68/PEI with the addition of Pluronic® P123 at different ratios, after filtration.
The particle size for all the ratios stayed under 200 nm, with the lowest value observed for the ratio 1:1. The PDI was mostly consistent, with a higher zeta potential value obtained for the ratios 1:1 and 2:1.
The morphology evaluation of the 2:1 ratio was performed by TEM (Figure 5). The nanoparticle size ranged from 68.05 to 111.35 nm. The nanoparticles appeared spherical in shape, with a slightly darker center and lighter shell.
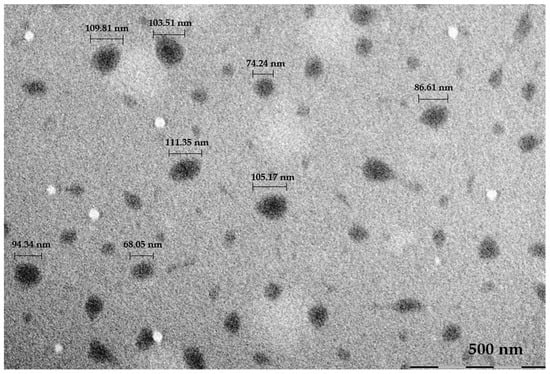
Figure 5.
TEM image of Pluronic®F68/PEI + Pluronic® P123 (2:1) with measurements of particle size.
4. Discussion
Cancer is one of the most common causes of mortality worldwide and the number of diagnosed patients has only been increasing over time [21]. OS is an aggressive bone tumor characterized by a high number of relapses, metastasis and a low patient survival rate. In consideration of this alarming panorama, investigations are being conducted to not only uncover novel treatment options, but to also overcome limitations to current therapeutics, improving their efficacy [22]. In particular, the nanomedicine and gene therapy fields have been extensively researched for this purpose. PMs are nanosystems that facilitate the targeted transportation of poorly water-soluble drugs to cancer cells and may be advantageous towards OS treatment [8]. PEI, a synthetic polymer with a cationic charge, is commonly used in the pharmaceutical field, being especially studied for the design of delivery vehicles for gene therapy. In its linear or branched form, it can form nanosized complexes with genetic material, conferring protection and allowing its intracellular delivery [23]. Its primary, secondary and tertiary amino groups concede a high transfection efficiency of a polymer-based delivery system. These characteristics make PEI a suitable non-viral gene carrier [24,25].
In this work, we decided to evaluate the potential of PEI conjugated in a PM formulation containing Pluronic® F68, as a potential therapeutic option for targeted delivery of genetic material to OS cells.
In order to conjugate Pluronic® F68 with PEI, this poloxamer needed to be activated first. This activation was confirmed by two different spectroscopy techniques, 1H-NMR and FTIR. The 1H-NMR spectra identified the appearance of new peaks due to the incorporation of olefinic acryloyl protons, indicative of the acrylate groups end-capped in Pluronic® F68. Additionally, there was a slight shift in the signal of the terminal CH2 PEO protons of the poloxamer. In the FTIR spectrum of Pluronic F68 diacrylate, a new bond could be detected, representative of the formation of the new ester bond.
After Pluronic® F68 was end-capped with acryloyl groups, PEI, dissolved in anhydrous dichloromethane, was able to conjugate with the co-polymer. DLS was performed following dialysis and lyophilization of the formulation, and the reduction in particle size observed after filtration may be associated with less unbounded PEI in the solution. The PDI also decreased to a more favorable value (0.2–0.3 or below) [26], and indicated a more homogeneous and stable solution. The zeta potential value is still relevant, as it was a positive value, despite the result being lower than the admitted value for a stable micelle, i.e., above +30 mV and lower than −30 mV [27]. The complexation of PEI with Pluronic F68 diacrylate was proved successful by 1H-NMR and FTIR spectroscopy. In the 1H-NMR spectrum for PEI, the peak corresponding to the CH2 groups of PEI, and the appearance of new peaks in F68/PEI spectrum in the same ppm zone and its absence in the diacrylate spectrum, was indicative of a successful conjugation of F68 with PEI. For the FTIR spectra, the new band appeared at 3400 cm−1, due to the N–H bond vibration, which unequivocally proves the presence of the conjugated PEI in the formulation.
The addition of Pluronic® P123 in the formulation was tested at different ratios and was able to lower the particle size, at any given ratio, to below 200 nm, and even when compared with the F68/PEI particle size measurement after filtration (see Table 1). It is also possible to assess that lower concentrations of Pluronic® P123, in the 1:1 and 2:1 ratios, led to a higher value of zeta potential. The 2:1 ratio was further characterized morphologically. The nanoparticle size observed by TEM was similar to, if slightly below, the mean results obtained through DLS. This indicates that the sample was uniform and presented a low aggregation. Regarding the appearance, the particles were spherical and presented a darker center and a lighter shell. The latter could be due to the hydrophilic chains of PEI.
5. Conclusions
In conclusion, Pluronic® F68 diacrylate was synthesized and characterized by 1H-NMR and FTIR spectroscopy. The complexation of PEI with the diacrylate was performed using anhydrous dichloromethane as the solvent and the particle size measurements were favorable (20–200 nm), but a slightly high polydispersity index and a low zeta potential were obtained. The latter value presented a challenge, as it influences the stability of the nanocomplex. The incorporation of Pluronic® P123, at different ratios, to the Pluronic® F68 reaction with PEI did ameliorate the polydispersity index and originated smaller particles than before, but the zeta potential remained lower than favorable (± 30 mV). The morphology characterization was in accordance with the results obtained in the DLS. The TEM results showed nanoparticles with a micellar structure and in the presence of PEI, a lighter hydrophilic shell was observed. In the future, we plan to explore the dual therapy ability of the nanosystem obtained and conjugate the latter with genetic material in the outer shell and a drug in the inner core, as a potential therapeutic strategy against OS cells.
Author Contributions
C.M., I.J. and A.F. conceived and designed the experiments; C.M. performed the experiments; E.T.d.S. and F.R. accompanied the chemical synthesis; A.F., E.T.d.S., F.R. and I.J. analyzed the data; I.J. contributed to analysis tools; C.M. wrote the paper; A.F. and F.V. revised the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work received financial support from National Funds (FCT/MEC, Fundação para a Ciência e Tecnologia/Ministério da Educação e Ciência) through the project UIDB/50006/2020, co-financed by the European Union (FEDER under the Partnership Agreement PT2020). It was also supported by the grant FCT PTDC/BTM-MAT/30255/2017 (POCI-01- 0145-FEDER-030255) from the Portuguese Foundation for Science and Technology (FCT) and the European Community Fund (FEDER) through the COMPETE2020 program.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest in the present work.
Abbreviations
The following abbreviations are used in this manuscript:
| DLS | Dynamic Light Scattering |
| EPR | Enhanced Permeation and Retention |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| 1H-NMR | Proton Nuclear Magnetic Resonance |
| OS | Osteosarcoma |
| PDI | Polydispersity Index |
| PEI | Polyethyleneimine |
| PEO | Poly(ethylene oxide) |
| PM | Polymeric Micelle |
| PPO | Poly(propylene oxide) |
| TEM | Transmission Electron Microscopy |
References
- Misaghi, A.; Goldin, A.; Awad, M.; Kulidjian, A. Osteosarcoma: A comprehensive review. SICOT-J 2018, 4, 12. [Google Scholar] [CrossRef]
- O’Day, K.; Gorlick, R. Novel therapeutic agents for osteosarcoma. Expert Rev. Anticancer Ther. 2009, 9, 511–523. [Google Scholar] [CrossRef] [PubMed]
- Hanh, P.L.T.; Novita, S.I.; Ying-Gui, Y.; Sang-Hyun, L.; Nayoung, J.; Seock, K.K.; Kyung, L.Y.; Young, K.H. Cancer stem cells (CSCs) in drug resistance and their therapeutic implications in cancer treatment. Stem Cells Int. 2018. [Google Scholar] [CrossRef]
- De Boer, J.P.; van Royen, B.; Helder, M. Mechanisms of therapy resistance in osteosarcoma: A review. Oncol. Discov. 2013, 1, 8. [Google Scholar] [CrossRef][Green Version]
- Sampson, V.B.; Yoo, S.; Kumar, A.; Vetter, N.S.; Kolb, E.A. MicroRNAs and potential targets in osteosarcoma: Review. Front. Pediatr. 2015, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Hu, H.; Qing, X.; Zhang, Z.-C.; Shao, Z.-W. Recent advances of drug delivery nanocarriers in osteosarcoma treatment. J. Cancer 2020, 11. [Google Scholar] [CrossRef]
- Savvidou, O.D.; Bolia, I.K.; Chloros, G.D.; Goumenos, S.; Sakellariou, V.I.; Galanis, E.; Papagelopoulos, P.J. Applied nanotechnology and nanoscience in orthopedic oncology. Orthopedics 2016, 39, 280–286. [Google Scholar] [CrossRef]
- Chow, E.K.H.; Ho, D. Cancer nanomedicine: From drug delivery to imaging. Sci. Transl. Med. 2013, 5, 1–12. [Google Scholar] [CrossRef]
- Tong, R.; Kohane, D.S. New Strategies in Cancer Nanomedicine. Annu. Rev. Pharmacol. Toxicol. 2016, 56, 41–57. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Amin, M.C.I.M.; Butt, A.M.; Amjad, M.W.; Kesharwani, P. Polymeric Micelles for Drug Targeting and Delivery. In Nanotechnology-Based Approaches for Targeting and Delivery of Drugs and Genes; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Deshmukh, A.S.; Chauhan, P.N.; Noolvi, M.N.; Chaturvedi, K.; Ganguly, K.; Shukla, S.S.; Nadagouda, M.N.; Aminabhavi, T.M. Polymeric micelles: Basic research to clinical practice. Int. J. Pharm. 2017, 532, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, S.S.; Kaur, S.; Tummala, H.; Sangamwar, A.T. Overcoming multiple drug resistance in cancer using polymeric micelles. Expert Opin. Drug Deliv. 2018, 15, 1127–1142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Huang, Y.; Li, S. Polymeric micelles: Nanocarriers for cancer-targeted drug delivery. AAPS PharmSciTech 2014, 15, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Hunter, A.C. Poloxamers and poloxamines in nanoparticle engineering and experimental medicine. Science 2000, 18, 2958–2964. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Alexandridis, P. Formulation of poloxamers for drug delivery. J. Funct. Biomater. 2018, 9, 11. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.K.; Lemieux, P.; Vinogradov, S.V.; Gebhart, C.L.; Guérin, N.; Paradis, G.; Bronich, T.K.; Alakhov, V.Y.; Kabanov, A.V. Evaluation of polyether-polyethyleneimine graft copolymers as gene transfer agents. Gene Ther. 2000, 7, 126–138. [Google Scholar] [CrossRef]
- Samal, S.K.; Dash, M.; Van Vlierberghe, S.; Kaplan, D.L.; Chiellini, E.; Van Blitterswijk, C.; Moroni, L.; Dubruel, P. Cationic polymers and their therapeutic potential. Chem. Soc. Rev. 2012, 41, 7147–7194. [Google Scholar] [CrossRef]
- Gáscon, A.R.; del Pozo-Rogríguez, A.; Solinís, M.Á. Non-Viral Delivery Systems in Gene Therapy; Intech: London, UK, 2013. [Google Scholar] [CrossRef]
- Magalhães, M.; Almeida, M.; Tavares-da-Silva, E.; Roleira, F.M.F.; Carla, V.; Joana, J.; Gonçalves, A.C.; Carvalho, R.A.; Santos, A.C.; Veiga, F.; et al. miR-145-loaded micelleplexes as a novel therapeutic strategy to inhibit proliferation and migration of osteosarcoma cells. Eur. J. Pharm. Sci. 2018, 123, 28–42. [Google Scholar] [CrossRef]
- Liao, J.; Jia, Y.; Wu, Y.; Shi, K.; Yang, D.; Li, P.; Qian, Z. Physical-, chemical-, and biological-responsive nanomedicine for cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2020, 12, e1581. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.H.; Jewell, B.E.; Gingold, J.; Lu, L.; Lee, D.F. Osteosarcoma: Molecular Pathogenesis and iPSC Modeling. Trends Mol. Med. 2017, 23, 737–755. [Google Scholar] [CrossRef]
- Pandey, A.P.; Sawant, K.K. Polyethylenimine: A versatile, multifunctional non-viral vector for nucleic acid delivery. Mater. Sci. Eng. C 2016, 68, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Demeneix, B.; Behr, J.P. Polyethylenimine (PEI). Adv. Genet. 2005, 53, 215–230. [Google Scholar] [CrossRef]
- Liang, W.; Gong, H.; Yin, D.; Lu, S.; Fu, Q. High-molecular-weight polyethyleneimine conjuncted pluronic for gene transfer agents. Chem. Pharm. Bull. 2011, 59, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. Methods for characterization of nanoparticles. Adv. Nanomed. Deliv. Ther. Nucleic Acids 2017, 44–58. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).