Abstract
Ziprasidone hydrochloride is an atypical antipsychotic agent with an anti-schizophrenic activity having less solubility and less bioavailability. The nasal route acts as a promising delivery route for CNS targeting drugs due to improved bioavailability and can reduce peripheral side effects. The main aim of the study is to prepare an in-situ nasal gel of ziprasidone-β-cyclodextrin for improvement in the bioavailability of the drug. The in-situ gel was optimized using box-Behnken design. The optimized formulation was evaluated for homogeneity, viscosity, gelation temperature, mucoadhesive strength, in-vitro permeation studies. The pharmacodynamic activity of in-situ nasal gel was checked using the locomotor activity model. According to the statistical analysis of the design expert software, all the models were found significant. The pharmacodynamic investigation of the cyclodextrin mediated in situ nasal gel showed improvement in drug activity compared to a drug, drug-complex given by oral route indicating the nasal delivery of antipsychotic drug improves its effect.
1. Introduction
Oral administration preferably used the route of administration for systemic effects. Drugs with low bioavailability were not significant when given by orally and endorsed to find a more significant systemic delivery route. Generally, the nasal cavity is used to treat a disease like rhinitis, migraine, cold, nasal congestion, and CNS disorder. Recently, several drugs have the capability of systemic bioavailability through the nasal route that has been approved. The nasal mucosa is a significant administration route to achieve a faster and higher drug absorption level due to its anatomy [1,2,3]. An in-situ gel is a type of dosage form in which the drug is in solution form before administration; after administration, it undergoes gelation to form a gel [2]. Nasal drug delivery offers a way to the brain that evades the blood-brain barrier [4]. Schizophrenia is a chronic disabling illness caused by an abnormal amount of certain neurotransmitters in the brain. Ziprasidone (ZPR) is a benzo thiazolyl piperazine derivative that belongs to the atypical class of antipsychotics to treat schizophrenia [5,6]. It is the fifth new atypical antipsychotic drug that acts as a selective monoaminergic antagonist with an affinity for serotonin Type-2 (5HT2), dopamine Type-2 (D2), 1 and 2 adrenergic, and H1 histaminergic receptor. ZPR has a pKa value of 6.5, and it is poorly water-soluble (0.007 mg/mL at 37 °C) and highly lipophilic [7].
Various techniques that can be used to enhance solubility are categorized as chemical and physical techniques. Employing cyclodextrin to improve drug solubility is a vital strategy [8]. Cyclodextrins are oligosaccharides that modify the physicochemical properties of the drugs. Cyclodextrin can accommodate the drug molecules within their cavity to form to yield inclusion complexes. Typical formulations prepared using the inclusion complexes possess a better absorption rate, shorter drug release time, and reduce toxicity-related side effects [9]. In the present study, prepared in-situ nasal gel of ziprasidone-β-cyclodextrin for improvement in the bioavailability of the drug.
2. Experiments
2.1. Materials
Ziprasidone hydrochloride with 99.7% purity was a gift sample from Macleods Pharma (Mumbai, India). β cyclodextrin (βCD) and Polaxomer 407 were procured from Gangwal chemicals (Mumbai, India) and BASF India, respectively. HPMC E5 and PEG 6000 were bought from S.D. Fine (Mumbai, India). Milli-Q water was used in the research. All the other reagents used were of analytical grade.
2.2. Method
In situ hydrogel can be instilled in the nasal cavity in the form of liquid and forms a gel making possible longer residence time and better drug release. Temperature is an important parameter to trigger a transition from liquid to gel state of in situ nasal gels. Poloxamer 407 was selected as a gelling agent due to the thermosensitive nature and polyethylene oxide, which induces gelation. Moreover, Pluronic is considered safe for use by the US FDA in medical applications. A formulation prepared using Pluronic F127 can be easily administered in the form of liquid and turns into a gel instantly at physiological temperature. The Drug-cyclodextrin complex was used as it showed better solubility [10].
- Optimization of the Concentrations of Lutrol F127, HPMC K4M, and PEG 400 Using the Box-Behnken Design
The in situ nasal gel formulation was optimized using 3 factors, 3-level Box-Behnken design. The concentration of the Poloxamer 407 (gelling agent), HPMCK4M (mucoadhesive polymer), and PEG 400 (Permeation enhancer) were considered as independent variables. Responses-The Gelation Temperature (R1) and Drug Release (R2) were considered dependent variables. The trial experiments were conducted based on three level-high, low, and medium for 3 factors. All analytical treatments performed using Design Expert 10 software manufactured by Stat Ease, Inc. Analysis of variance was carried out to determine the significance of the fitted equation.
- Evaluation of In-Situ Gel
2.3. Physical and Rheological Study
The optimized formulation was tested for homogeneity by visual inspection after the gel was set in the container. It was tested for the presence of the aggregates and appearance. The in-situ gel’s apparent pH was determined using the pH meter at room temperature. The solution to gel transition was determined using the Visual Tube Inversion Method. A mucoadhesive test is performed to make sure that the gel inside the nose adheres to the nasal mucosa of the nasal cavity. Sheep nasal mucosa was used for this purpose. The viscosity of the gel formulation was measured using the Brookfield Viscometer (Model No. LVDV II + PRO). The mucoadhesive force was measured using the sheep nasal mucosa and phosphate buffer 6.8 as the moistening fluid [11].
2.4. In Vitro Diffusion Studies
In vitro Diffusion studies were performed in triplicates by taking gel equivalent to 5 mg of pure ZPR with the dialysis membrane. Diffusion studies were performed in phosphate buffer (pH 6.4) with 1% SLS using the Franz diffusion assembly at 37 ± 0.5 °C and stirred at 50 rpm. At predetermined time intervals, aliquots of 2 mL were withdrawn and filtered through the Whatman filter paper. Sink conditions were maintained by replacing equal amounts of fresh media. Further, samples were analyzed spectrophotometrically. The graph of percent drug release of ZPR against time (minutes) was plotted.
2.5. Pharmacodynamic Investigation of Cyclodextrin Mediated In Situ Nasal Gel Using Animal Models
Locomotor activity was carried out on Wistar rats. Animals were divided into 5 groups containing 6 animals each. Except for Group I, psychosis was induced in all the groups via the intraperitoneal route for 5 days. After this, Group III was treated with ZPR suspension (in 0.1% CMC solution), which was administered orally. Group IV has treated with ZPR βCD solution administered orally. Group V received the in situ nasal gel via the nasal route for 10 days. After treatment for 10 days as per the above groups, the locomotor activity was checked for 5 min using an actophotometer. A digital photoactometer was used to assess locomotor activity in rats. Rats were placed individually inside the activity box, and the test was started after 1 min of the acclimatization period and concluded after 5 min. Scores based on the number of photobeam interruption in a five-minute run were recorded. The activity box was wiped with dilute (35% v/v) ethanol and cleaned free of any fecal matter before placing the next rat.
3. Results and Discussion
3.1. Subsection
- Optimization of the Concentrations of Lutrol F127, HPMC K4M, and PEG 400 Using the Box-Behnken Design
In the present study, various formulations were prepared based on gelling agent concentration, mucoadhesive polymer, and the permeation enhancer. Box-behken Design of Experiment was adopted, which gave a trial of 17 runs. The concentrations of Poloxamer 407, HPMC K4M, and PEG 400 were considered independent variables A, B, and C, respectively. The responses R1 and R2 were considered as dependent variables. ANOVA test for the Response R1 and R2 showed a p-value of less than 0.001; thus, the model was significant (Table 1). The factorial equation for gelation temperature and drug release revealed a good correlation coefficient (0.99) and the Model F value of 154.84 and 6.75, respectively, which implies the model is significant (Figure 1). Hence, the mucoadhesive polymer concentration, gelling agent, and the permeation enhancer affected the gelation temperature. According to Equations (1) and (2), all terms in the equation are negative, which indicates the effect of variables is antagonistic on the responses R1 and R2. Thus it depicted that as the concentration of poloxamer 407 increases, the gelation temperature decreases. Also, the drug release decreases due to the increase in the viscosity, which inhibits the permeation.

Table 1.
ANOVA results for the quadratic model for the response gelation temperature (R1) and drug release at the end of 5 h (R2).
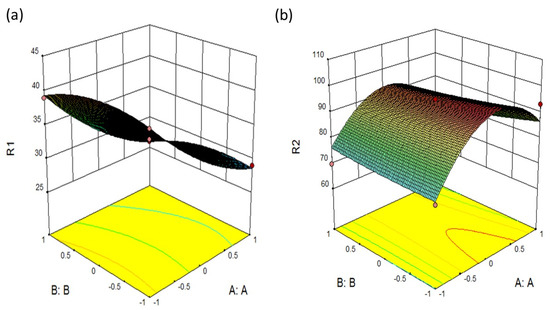
Figure 1.
3D surface plot presenting the interaction between the PLX407 and HPMC affecting (a) gelation temperature (b) drug release.
- Evaluation of In-Situ Gel
3.2. Physical and Rheological Study
The optimized formulation was homogenous, clear, and transparent. The apparent pH in the solution state is an essential criterion for its administration intranasally. The nasal mucosa can tolerate solutions within the range of 3–10. The pH of the optimized formulation was found to be 5.5 ± 0.58. The temperature of the nasal cavity ranges between 34 °C and 35 °C. The gelation temperature of the optimized formulation ranges within the desired temperature (35.23 °C ± 1.20) for intranasal administration. Mucoadhesive strength was determined in terms of detachment stress, and it was predominantly dependent on the level of HPMCK4M. Consequently, as the level of HPMC K4M increased, the mucoadhesive strength also increased. The inclusion of HPMC K4M in the in situ gelling systems is favored by its wetting properties that permit intimate contact of the dosage form with nasal tissue. The mucoadhesive force is more because it can prevent the gelled solution from coming out of the nose and lead to prolonged retention and increased absorption across mucosal tissues. However, excessive mucoadhesive force (i.e., greater than 10,000 dyne/cm2) gel can damage the nasal mucosal membrane. The mucoadhesive strength and viscosity of optimized formulation were 1320 dynes/cm2 and 1100cp at 10RPM, respectively. It was neither too high to form a very stiff gel nor low viscosity, which would drain off from the nasal mucosa.
3.3. In Vitro Diffusion Studies
The optimized batches showed a good diffusion release profile in Figure 2. The inclusion complexes incorporated into the in situ nasal gel exhibited a release of 92.85% at the end of 5 h (Figure 2). Thus more than 75% of the drug was released at the end of 3 h. Consequently, 90% of the drug permeated across the nasal mucosa within 5 h for formulations. The dissolving property of β-CD increased gel porosity and facilitated drug permeation by bringing the drug through the aqueous barrier to the lipotropic surface of the biological membrane [12].
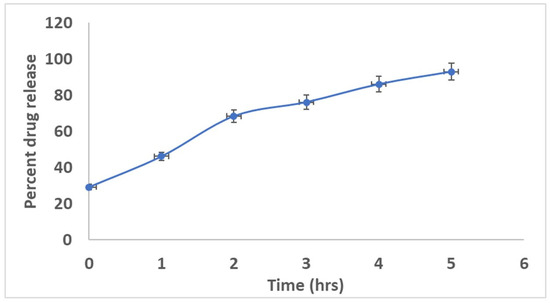
Figure 2.
Graph of in-vitro diffusion of the drug from in situ gel against time (Mean ± SD).
3.4. Pharmacodynamic Investigation of Cyclodextrin Mediated In Situ Nasal Gel Using Animal Models
Antipsychotic models have been done to evaluate the pharmacodynamics action of solubility enhancement formulations. The drug is a BCS II drug with low solubility and high permeability, so the solubility enhancement was done and tested in animal models. An animal study (locomotor activity) was done to check the nasal formulation’s effectiveness in solubility enhancement. On induction of psychosis for 5 days, the locomotor counts had increased to 276 from 192. On treatment for 10 days, it was observed that the locomotor counts decreased for the groups treated by using oral suspension and the in situ nasal formulation. The in situ nasal gel (*** p 0.05) showed a very significant response compared to –ve and +ve control using Durnet Test. The group treated with nasal formulation showed decreased locomotor activity, indicating the formulation’s effectiveness due to the faster delivery via the olfactory nerve across the blood-brain barrier. The oral administrated groups with ZPR-βCD suspension (*** p < 0.05) showed a significant response compared to –ve and +ve control using the Tukey’s test. The response obtained via the oral route was less significant as compared to the nasal route. Thus, the nasal route serves as an effective route for the treatment of schizophrenia, and the solubility of the formulation was enhanced successfully using beta-cyclodextrin (Figure 3).
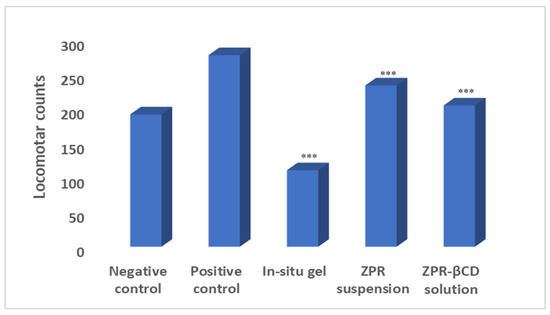
Figure 3.
Effect of induction and treatment in assessing the locomotor activity (Mean ± SD, *** p < 0.0001 when compared with −ve and +ve control).
3.5. Mathematical Components
R1 = +3360000 − 6.5000*A − 1.12500*B − 0.12500*C − 0.25000*A*B − 1.25000*A*C + 0.50000*B*C + 1.70000*A2 − 1.55000*B2 − 0.55000*C2,
R2 = +94.54 + 3.38*A − 1.8200*B − 3.82000*C − 2.45000*A*B −
2.69000*A*C + 1.18500*B*C − 16.10100*A2 + 0.52900*B2 − 6.05100*C
2.69000*A*C + 1.18500*B*C − 16.10100*A2 + 0.52900*B2 − 6.05100*C
4. Conclusions
The solubility of ziprasidone was efficaciously enhanced by its inclusion complex with β-cyclodextrin and was formulated as in-situ nasal gel. The optimized formulation comprising drug with β-cyclodextrin showed significant release and mucoadhesive strength to confirm suitable residence time at the site of action. Pharmacodynamic investigation of the cyclodextrin mediated in-situ nasal gel concluded that the nasal route of delivery helps to overcome the hepatic metabolism, dose-related side effects are reduced, provides rapid onset of action to target the CNS for treatment of schizophrenia.
Author Contributions
V.L. and S.K. conceived and designed the experiments; S.K. performed the experiments; V.L. and S.K. analyzed the data; V.L. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The experimental protocol (Protocol number: CPCSEA/IAEC/P-05/2017) was approved by Institutional Animal Ethics Committee (IAEC) which was constituted as per CPCSEA (Committee for the Purpose of Control and Supervision of Experiments on Animals), Govt. of India guidelines.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are thankful to Macleodes Pharma and Gangawal Chemicals to provide gift samples of Ziprasidone and β-cyclodextrin respectively.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ZPR | Ziprasidone |
| β CD | β cyclodextrin |
References
- Manjunath, K.; Kulkarni, S. Development Characterization and Evaluation of Nasal in situ Gel containing Anti-Asthmatic Drug. Int. J. Pharma Res. Health Sci. 2019, 7, 3001–3006. [Google Scholar] [CrossRef]
- Palhal, A. In-situ nasal gel: Modernistic advancement in drug delivery. World J. Pharm. Res. 2017, 566–577. [Google Scholar] [CrossRef]
- Sabale, A.; Kulkarni, A.; Sabale, A. Nasal In Situ Gel: Novel Approach for Nasal Drug Delivery. J. Drug Deliv. Ther. 2020, 10, 183–197. [Google Scholar] [CrossRef]
- Sharma, K. Recent advancement in drug delivery system for brain: An overview. World J. Pharm. Pharm. Sci. 2017, 292–305. [Google Scholar] [CrossRef]
- Lally, J.; MacCabe, J. Antipsychotic medication in schizophrenia: A review. Br. Med. Bull. 2015, 114, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Daniel, D.G.; Zimbroff, D.L.; Potkin, S.G.; Reeves, K.R.; Harrigan, E.P.; Lakshmi Narayanan, M.; Ziprasidone Study Group. Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: A 6-week placebo-controlled trial. Neuropsychopharmacology 1999, 20, 491–505. [Google Scholar] [CrossRef]
- Gauniya, A.; Mazumder, R.; Pathak, K. Formulation, optimization, and characterization of ziprasidone nanocrystals prepared by media milling technique. Int. J. Pharm. Pharm. Sci. 2015, 7, 146–150. [Google Scholar]
- Leuner, C.; Dressman, J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000, 50, 47–60. [Google Scholar] [CrossRef]
- Conceicao, J.; Adeoye, O.; Cabral-Marques, H.M.; Lobo, J. Cyclodextrins as Drug Carriers in Pharmaceutical Technology: The State of the Art. Curr. Pharm. Des. 2018, 24, 1405–1433. [Google Scholar] [CrossRef] [PubMed]
- Londhe, V.; Krishnan, S. Effect of inclusion of citric acid and Lutrol® F-68 on ziprasidone and β-cyclodextrin complexation: Characterization, solubility and dissolution studies. Eur. J. Chem. 2020, 11, 4. [Google Scholar] [CrossRef]
- Marzouk, M.; Osman, D.; Abd El-Fattah, A. Formulation and in vitro evaluation of a thermoreversible mucoadhesive nasal gel of itopride hydrochloride. Drug Dev. Ind. Pharm. 2018, 44, 1857–1867. [Google Scholar] [CrossRef]
- Masson, M.; Loftsson, T.; Masson, G.; Stefansson, E. Cyclodextrins as permeation enhancers; some theoretical evaluations and in-vitro testing. J. Control. Release 1999, 59, 107–118. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).