Design, Fabrication and Characterization of PVA/PLGA Electrospun Nanofibers Carriers for Improvement of Drug Delivery of Gliclazide in Type-2 Diabetes †
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Preparation of Spinning Solution for Gliclazide Electrospun Nanofibers
2.3. Electrospinning for Gliclazide Nanofibers
2.4. Characterizations of Gliclazide Nanofibers
2.4.1. Drug Content Study
2.4.2. Solubility Test
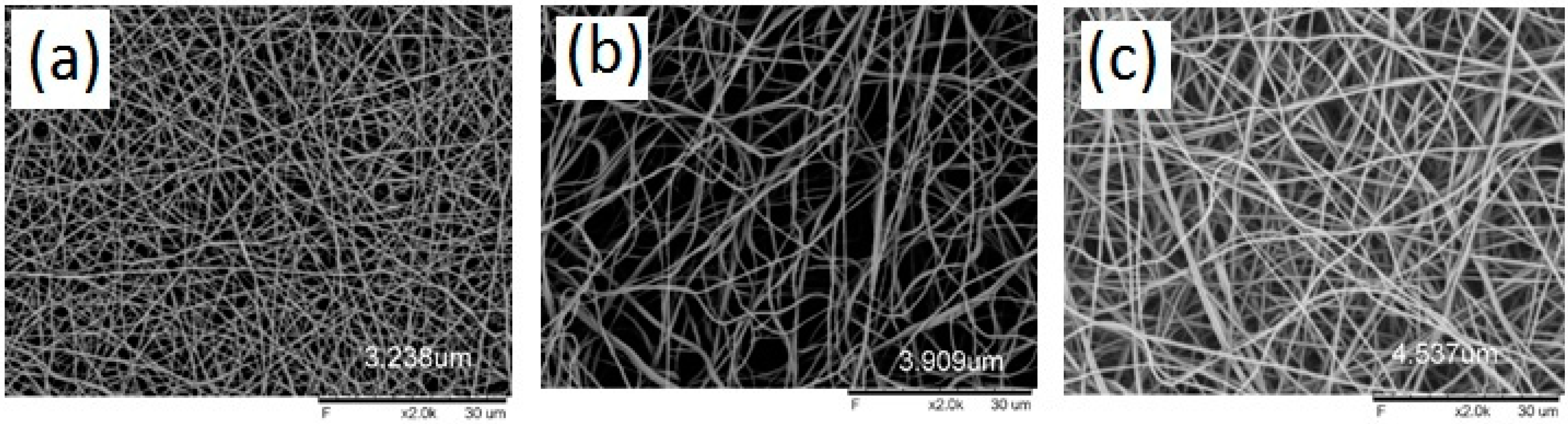
2.4.3. Scanning Electron Microscopy (SEM) Studies
2.4.4. In Vitro Drug Release Studies and Drug Release Kinetic Studies
2.4.5. Differential Scanning Calorimeter (DSC)
2.4.6. Fourier Transform Infrared Spectroscopy (FT-IR)
3. Results and Discussion
4. Conclusions
Author Contributions
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Balogh, A.; Farkas, B.; Faragó, K.; Farkas, A.; Wagner, I.; Verreck, G.; Nagy, Z.K.; Marosi, G. Melt-blown and electrospun drug-loaded polymer fiber mats for dissolution enhancement: A comparative study. J. Pharm. Sci. 2015, 104, 1767–1776. [Google Scholar] [CrossRef] [PubMed]
- Payab, S.; Davaran, S.; Tanhaei, A.; Fayyazi, B.; Jahangiri, A.; Farzaneh, A.; Adibkia, K. Triamcinolone acetonide-Eudragit® RS100 nanofibers and nanobeads: Morphological and physicochemical characterization. Artif. Cells Nanomed. Biotechnol. 2016, 44, 362–369. [Google Scholar] [CrossRef]
- Panda, B.P.; Krishnamoorthy, R.; Bhattamisra, S.K.; Shivashekaregowda, N.K.H.; Seng LBin Patnaik, S. Fabrication of Second Generation Smarter PLGA Based Nanocrystal Carriers for Improvement of Drug Delivery and Therapeutic Efficacy of Gliclazide in Type-2 Diabetes Rat Model. Sci. Rep. 2019, 9, 17331. [Google Scholar] [CrossRef]
- Sarkar, A.; Tiwari, A.; Bhasin, P.S.; Mitra, M. Pharmacological and pharmaceutical profile of gliclazide: A review. J. Appl. Pharm. Sci. 2011, 1, 11–19. [Google Scholar]
- Huang, W.; Yang, Y.; Zhao, B.; Liang, G.; Liu, S.; Liu, X.L.; Yu, D.G. Fast dissolving of ferulic acid via electrospun ternary amorphous composites produced by a coaxial process. Pharmaceutics 2018, 10, 115. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, B.; Aleem, A.; Najabat Ali, M.; Mir, M. Review of the fabrication techniques and applications of polymeric electrospun nanofibers for drug delivery systems. J. Drug Deliv. Sci. Technol. 2018, 48, 82–87. [Google Scholar] [CrossRef]
- Vashisth, P.; Raghuwanshi, N.; Srivastava, A.K.; Singh, H.; Nagar, H.; Pruthi, V. Ofloxacin loaded gellan/PVA nanofibers-Synthesis, characterization and evaluation of their gastroretentive/mucoadhesive drug delivery potential. Mater. Sci. Eng. C 2017, 71, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; Davaran, S.; Fayyazi, B.; Tanhaei, A.; Payab, S.; Adibkia, K. Application of electrospraying as a one-step method for the fabrication of triamcinolone acetonide-PLGA nanofibers and nanobeads. Colloids Surf. B Biointerfaces 2014, 123, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Poller, B.; Strachan, C.; Broadbent, R.; Walker, G.F. A minitablet formulation made from electrospun nanofibers. Eur. J. Pharm. Biopharm. 2017, 114, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Illangakoon, U.E.; Nazir, T.; Williams, G.R.; Chatterton, N.P. Mebeverine-loaded electrospun nanofibers: Physicochemical characterization and dissolution studies. J. Pharm. Sci. 2014, 103, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, W.; Wang, G.; Qu, Y.L.; Yu, D.G. Electrospun 4th-Generation Solid Dispersions of Poorly Water-Soluble Drug Utilizing Two Different Processes. J. Nanomater. 2018, 2018, 2012140. [Google Scholar] [CrossRef]
- Jahangiri, A.; Barzegar-Jalali, M.; Javadzadeh, Y.; Hamishehkar, H.; Adibkia, K. Physicochemical characterization of atorvastatin calcium/ezetimibe amorphous nano-solid dispersions prepared by electrospraying method. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.P.; Krishnamoorthy, R.; Shivashekaregowda, N.K.H.; Patnaik, S. Influence of Poloxamer-188 on design and development of second generation PLGA Nanocrystals of Metformin Hydrochloride. Nano Biomed. Eng. 2018, 10, 334–343. [Google Scholar] [CrossRef]
- Baishya, H. Application of Mathematical Models in Drug Release Kinetics of Carbidopa and Levodopa ER Tablets. J. Dev. Drugs 2017, 6, 1–8. [Google Scholar] [CrossRef]
- Wójcik-Pastuszka, D.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osiński, B.; Musiał, W. Evaluation of the release kinetics of a pharmacologically active substance from model intra-articular implants replacing the cruciate ligaments of the knee. Materials 2019, 12, 1202. [Google Scholar] [CrossRef]
- Javadzadeh, Y.; Ahadi, F.; Davaran, S.; Mohammadi, G.; Sabzevari, A.; Adibkia, K. Preparation and physicochemical characterization of naproxen-PLGA nanoparticles. Colloids Surf. B Biointerfaces 2010, 81, 498–502. [Google Scholar] [CrossRef] [PubMed]
- Panda, B.P. Impact of statistical central composite face centered design approach on method and process optimization of metformin hydrochloride loaded plga nanoformulation. Micro Nanosyst. 2017, 9, 55–71. [Google Scholar] [CrossRef]
- Fonseca, C.; Simões, S.; Gaspar, R. Paclitaxel-loaded PLGA nanoparticles: Preparation, physicochemical characterization and in vitro anti-tumoral activity. J. Control. Release 2002, 83, 273–286. [Google Scholar] [CrossRef]
- Panda, B.P.; Jessica, G. Extraction and Performance Evaluation of Salvia hispanica Mucilage as Natural Disintegrants for Optimization of Pyrilamine Maleate Fast Dissolving Tablets. Nat. Prod. J. 2015, 1, 288–298. [Google Scholar] [CrossRef]


| Formulation Code | % w/v | Nanofiber Fabrication Parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| Gliclazide | PVA | PLGA | Metallic Needle (Inner Diameter) in mm | Flow Rate in mL/h | Voltage Applied in kV | Distance between Needle’s Tip and Collector in cm | Ambient Condition (Temperature °C) | |
| BNF0 (BlankPVA Nanofiber) | 0 | 10 | 0 | 0.33 | 1 | 19 | 18 | 22 ± 1 |
| GLZNF1 | 0.1 | 10 | 0 | 0.33 | 1 | 19 | 18 | 22 ± 1 |
| GLZNF2 | 0.1 | 10 | 0.05 | 0.33 | 1 | 19 | 18 | 22 ± 1 |
| SGNCF3 | 0.1 | 10 | 0.10 | 0.33 | 1 | 19 | 18 | 22 ± 1 |
| GLZNF4 | 0.1 | 10 | 0.15 | 0.33 | 1 | 19 | 18 | 22 ± 1 |
| GLZNF5Caps (GLZNF2 in capsule) | 0.1 | 10 | 0.05 | 0.33 | 1 | 19 | 18 | 22 ± 1 |
| Formulation Code | Drug Content (%) | Folds Increase in Solubility ± SD (Compared with Pure Gliclazide) | Average Fiber Diameter ± SD (µm) (from SEM Studies) |
|---|---|---|---|
| BNF0 (Blank PVA Nanofiber) | - | - | 3.238 ± 0.47 |
| GLZNF1 | 96.82 ± 1.69 | 4.17 ± 1.04 | 3.909 ± 1.53 |
| GLZNF2 | 98.36 ± 0.87 | 2.84 ± 1.75 | 4.537 ± 1.88 |
| SGNCF3 | 96.24 ± 2.50 | 2.25 ± 0.28 | 5.261 ± 1.45 |
| GLZNF4 | 96.13 ± 1.14 | 1.84 ± 0.17 | 5.537 ± 2.73 |
| GLZNF5Caps | 97.86 ± 1.36 | 2.84 ± 1.94 | 4.537 ± 1.88 |
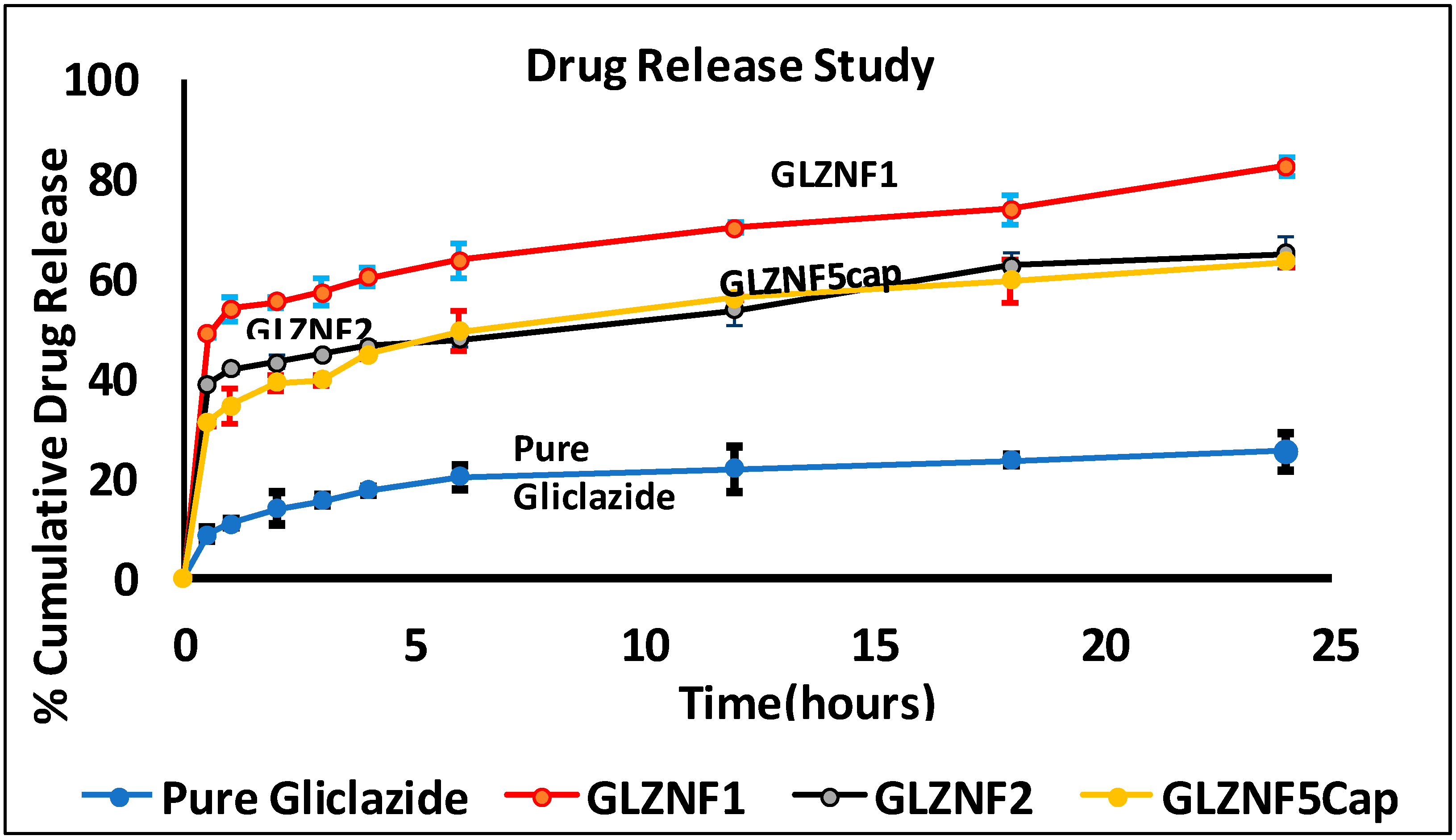
| Time (h) | % Cumulative Drug Release ± SD | |||||
|---|---|---|---|---|---|---|
| Pure Gliclazide | GLZNF1 (10% PVA) | GLZNF2 (Optimized Formulation) | GLZNF3 | GLZNF4 | GLZNF5Cap (Optimized Formulation in Capsule) | |
| 0.5 | 8.62 ± 1.2 | 48.87 ± 0.79 | 38.35 ± 0.49 | 27.306 ± 2.9 | 14.130 ± 0.79 | 31.16 ± 0.94 |
| 1 | 10.79 ± 0.82 | 53.84 ± 2.58 | 41.72 ± 0.75 | 30.690 ± 1.5 | 16.254 ± 1.42 | 34.37 ± 3.45 |
| 2 | 13.714 ± 3.4 | 55.12 ± 1.02 | 43.00 ± 1.38 | 31.968 ± 2.2 | 17.964 ± 1.74 | 38.96 ± 1.62 |
| 3 | 15.40 ± 1.3 | 57.25 ± 2.9 | 44.69 ± 0.46 | 34.092 ± 1.4 | 19.638 ± 0.56 | 39.6 ± 1.1 |
| 4 | 17.53 ± 0.8 | 60.22 ± 1.86 | 46.40 ± 0.9 | 35.370 ± 2.46 | 20.934 ± 2.8 | 44.69 ± 0.67 |
| 6 | 20.08 ± 2.6 | 63.62 ± 3.43 | 47.68 ± 1.41 | 37.908 ± 3.02 | 22.194 ± 2.41 | 49.37 ± 3.93 |
| 12 | 21.77 ± 4.57 | 69.99 ± 1.06 | 53.62 ± 3.28 | 41.724 ± 1.98 | 24.750 ± 0.97 | 56.16 ± 0.47 |
| 18 | 23.47 ± 1.25 | 73.81 ± 2.93 | 62.53 ± 2.32 | 45.972 ± 2.45 | 28.152 ± 0.25 | 59.56 ± 4.24 |
| 24 | 25.18 ± 3.8 | 82.47 ± 2.01 | 65.08 ± 3.08 | 49.806 ± 2.85 | 31.536 ± 1.47 | 63.37 ± 1.12 |
| Gliclazide Nanofiber Formulation | Zero-Order Plots | First-Order Plots | Higuchi’s Plots | Korsmeyer–Peppas Plots | ||
|---|---|---|---|---|---|---|
| Correlation Coefficient (R02) | Correlation Coefficient (R12) | Correlation Coefficient (R2) | Correlation Coefficient (Rk2) | Diffusional Exponent (n) | Type of Release | |
| GLZNF1 | 0.478 | 0.7627 | 0.712 | 0.2041 | 0.453 | Fickian diffusion |
| GLZNF2 | 0.523 | 0.7114 | 0.727 | 0.2233 | 0.4498 | Fickian diffusion |
| SGNCF3 | 0.534 | 0.6554 | 0.7681 | 0.2381 | 0.4292 | Fickian diffusion |
| GLZNF4 | 0.5005 | 0.5499 | 0.7914 | 0.2804 | 0.3938 | Fickian diffusion |
| GLZNF5Cap | 0.5996 | 0.7586 | 0.8499 | 0.266 | 0.4834 | Fickian diffusion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panda, B.P.; Wei, M.X.; Shivashekaregowda, N.K.H.; Patnaik, S. Design, Fabrication and Characterization of PVA/PLGA Electrospun Nanofibers Carriers for Improvement of Drug Delivery of Gliclazide in Type-2 Diabetes. Proceedings 2021, 78, 14. https://doi.org/10.3390/IECP2020-08689
Panda BP, Wei MX, Shivashekaregowda NKH, Patnaik S. Design, Fabrication and Characterization of PVA/PLGA Electrospun Nanofibers Carriers for Improvement of Drug Delivery of Gliclazide in Type-2 Diabetes. Proceedings. 2021; 78(1):14. https://doi.org/10.3390/IECP2020-08689
Chicago/Turabian StylePanda, Bibhu Prasad, Mok Xiu Wei, Naveen Kumar Hawala Shivashekaregowda, and Sujata Patnaik. 2021. "Design, Fabrication and Characterization of PVA/PLGA Electrospun Nanofibers Carriers for Improvement of Drug Delivery of Gliclazide in Type-2 Diabetes" Proceedings 78, no. 1: 14. https://doi.org/10.3390/IECP2020-08689
APA StylePanda, B. P., Wei, M. X., Shivashekaregowda, N. K. H., & Patnaik, S. (2021). Design, Fabrication and Characterization of PVA/PLGA Electrospun Nanofibers Carriers for Improvement of Drug Delivery of Gliclazide in Type-2 Diabetes. Proceedings, 78(1), 14. https://doi.org/10.3390/IECP2020-08689





