Sub-100 nm Chitosan-Triphosphate-DNA Nanoparticles for Delivery of DNA Vaccines †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
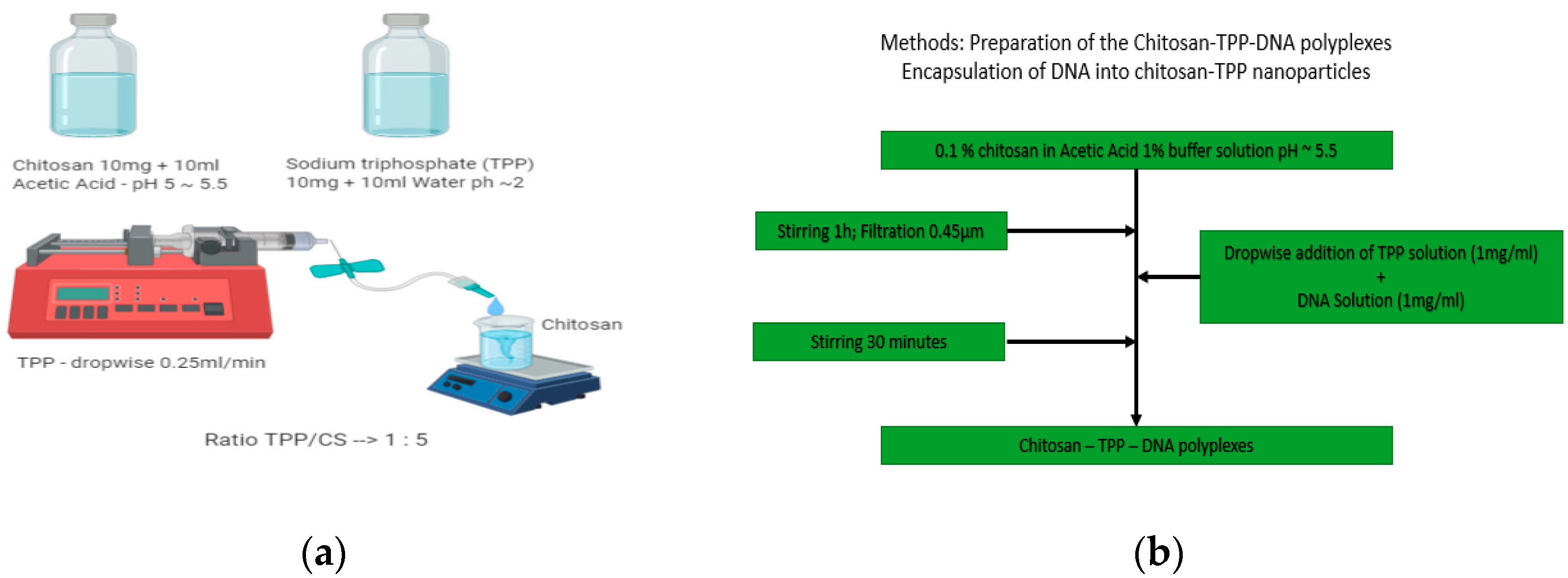
2.2. Methods
2.2.1. Ionotropic Gelation
2.2.2. Encapsulation Efficiency
2.2.3. Particle Size Determination
3. Results
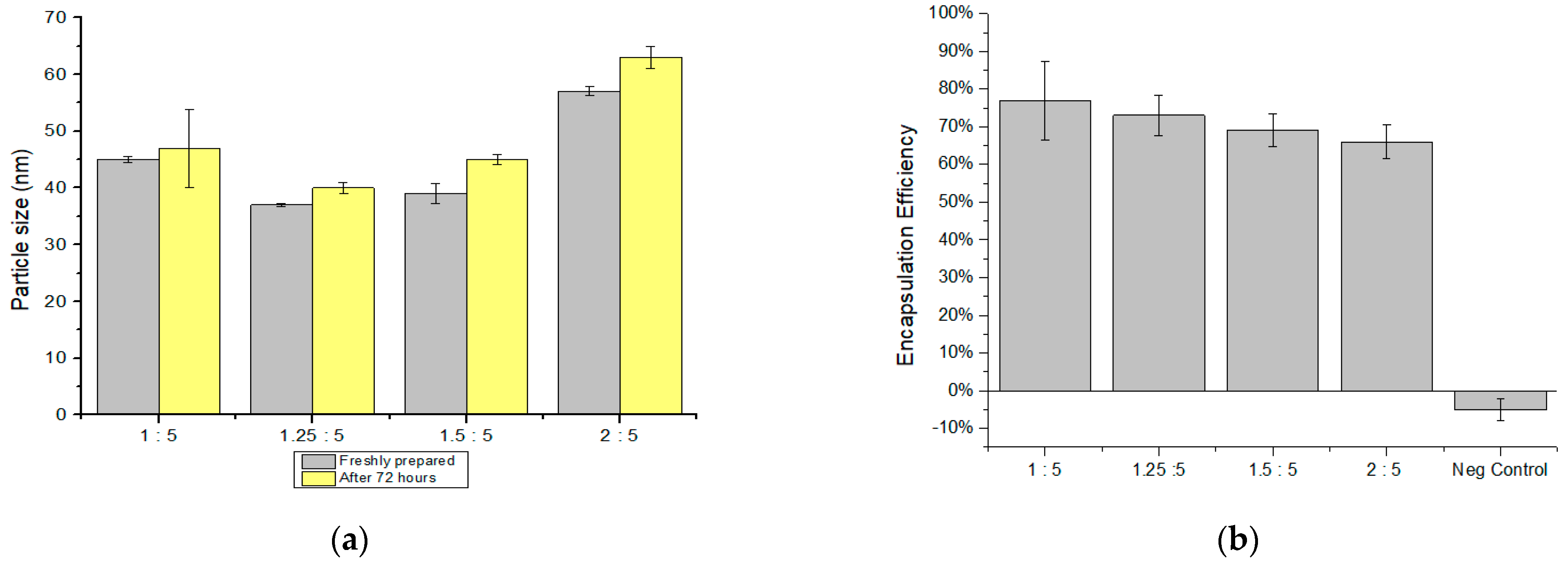
3.1. Influence of Changing the TPP/CS Ratio on NPs Size and dEE
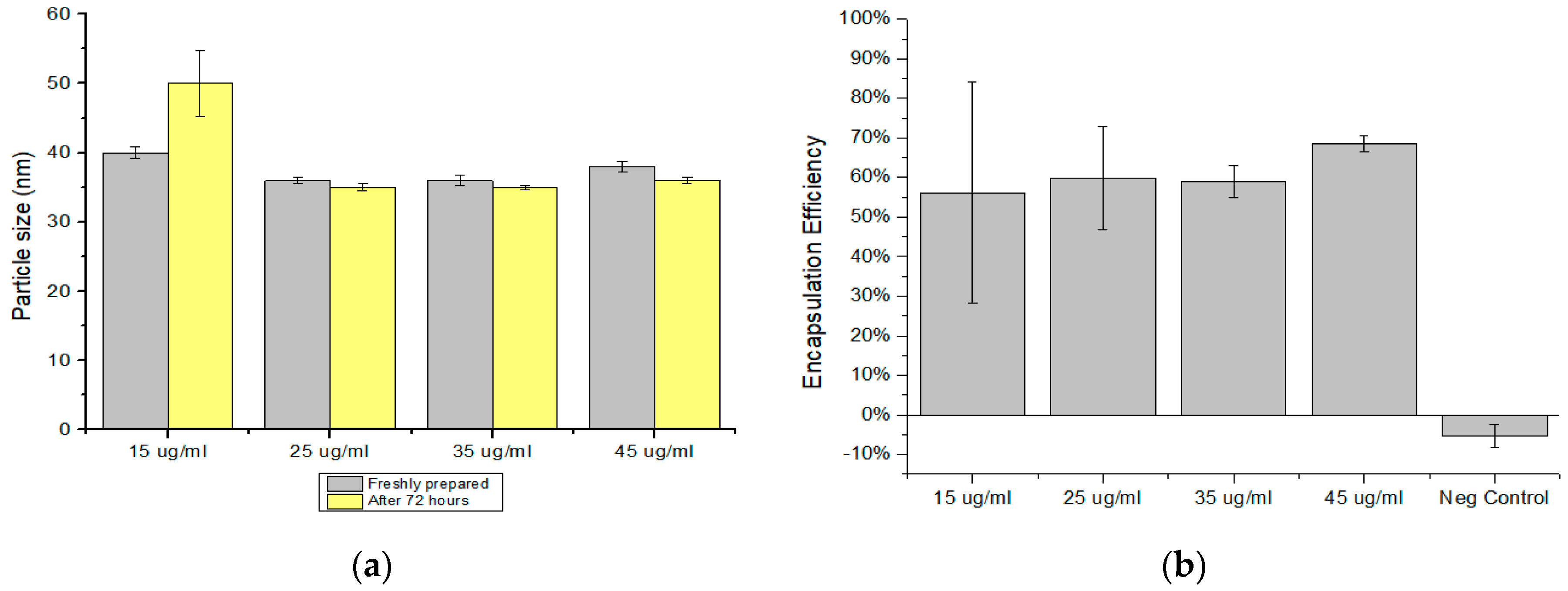
3.2. Influence of Changing the DNA Concentration in NP Size and dEE
4. Discussion and Future Work
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Nagai, H.; Kim, Y.H. Cancer prevention from the perspective of global cancer burden patterns. J. Thorac. Dis. 2017, 9, 448–451. [Google Scholar] [CrossRef] [PubMed]
- Doorbar, J.; Egawa, N.; Griffin, H.; Kranjec, C.; Murakami, I. Human papillomavirus molecular biology and disease association. Rev. Med. Virol. 2015, 25, 2–23. [Google Scholar] [CrossRef] [PubMed]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Amin, T.; Sampat, B.; Cook-Deegan, R.; Chandrasekharan, S. Intellectual property, technology transfer and manufacture of low-cost HPV vaccines in India [published correction appears in Nat Biotechnol. 2012 Feb;30(2):193]. Nat. Biotechnol. 2010, 28, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.M.; Queiroz, J.A.; Sousa, F.; Sousa, A. Cervical cancer and HPV-Infection: Ongoing therapeutic research to counteract the action of E6 and E7 oncoproteins. Drug Discov. Today 2019, 24, 2044–2057. [Google Scholar] [CrossRef] [PubMed]
- Ha, S.; Jeon, B.; Youn, J.; Kim, S.; Cho, S.; Sung, Y. Protective effect of DNA vaccine during chemotherapy on reactivation and reinfection of Mycobacterium tuberculosis. Gene Ther. 2005, 12, 634–638. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Xin, K.Q.; Haruki, A.; Kawamoto, S.; Kojima, Y.; Hirahara, F.; Okada, H.; Klinman, D.; Hamajima, K. Transplacental genetic immunization after intravenous delivery of plasmid DNA to pregnant mice. J. Immunol. 2001, 167, 5478–5484. [Google Scholar] [CrossRef] [PubMed]
- Smith, H.A.; Klinman, D.M. The regulation of DNA vaccines. Curr. Opin. Biotechnol. 2001, 12, 299–303. [Google Scholar] [CrossRef]
- Kutzler, M.A.; Weiner, D.B. DNAvaccines: Ready for prime time? Nat. Rev. Genet. 2008, 9, 776–788. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.J.; Schneider, J. Plasmid DNA and viral vector-based vaccines for the treatment of cancer. Vaccine 2007, 25 (Suppl. 2), B24–B34. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Arun Kumar, S.; Jhan, Y.Y.; Bishop, C.J. Engineering DNA vaccines against infectious diseases. Acta Biomater. 2018, 80, 31. [Google Scholar] [CrossRef]
- Mukherjee, S.; Ray, S.; Thakur, R.S. Solid lipid nanoparticles: A modern formulation approach in drug delivery system. Indian J. Pharm. Sci. 2009, 71, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Panyam, J.; Labhasetwar, V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv. Drug Deliv. Rev. 2003, 55, 329–347. [Google Scholar] [CrossRef]
- López-León, T.; Carvalho, E.L.; Seijo, B.; Ortega-Vinuesa, J.L.; Bastos-González, D. Physicochemical characterization of chitosan nanoparticles: Electrokinetic and stability behavior. J. Colloid Interface Sci. 2005, 283, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, M.; Saimoto, H.; Usui, H.; Okamoto, Y.; Minami, S.; Shigemasa, Y. Biological activities of carbohydrate-branched chitosan derivatives. Biomacromolecules 2001, 2, 1133–1136. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Tsushima, Y.; Morimoto, M.; Saimoto, H.; Okamoto, Y.; Minami, S.; Shigemasa, Y. Biological activity of chitosan-sugar hybrids: Specific interaction with lectin. Polym. Adv. Technol. 2000, 11, 176–179. [Google Scholar] [CrossRef]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Özbaş-Turan, S.; Akbuğa, J. Plasmid DNA-loaded chitosan/TPP nanoparticles for topical gene delivery. Drug Deliv. 2011, 18, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, W.; Zhou, Q.; Liu, S.; Xu, W.; Sun, T.; Liang, Y. Establishing Gene Delivery Systems Based on Small-Sized Chitosan Nanoparticles. J. Ocean Univ. China 2018, 17, 1253–1260. [Google Scholar] [CrossRef]
- Huang, K.S.; Sheu, Y.R.; Chao, I.C. Preparation and Properties of Nanochitosan. Polym. Plast. Technol. Eng. 2009, 48, 1239–1243. [Google Scholar] [CrossRef]
| TPP/CS Ratio | Z-Average Size (nm) | Polydispersity Index (PDI) | Z—Average Size after 72 h (nm) | (PDI) after 72 h | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|
| 1:5 | 45 ± 0.5 | 0.55 ± 0.01 | 47 ± 6.9 | 0.51 ± 0.006 | 77 ± 10 |
| 1.25:5 | 37 ± 0.3 | 0.50 ± 0.03 | 40 ± 0.9 | 0.48 ± 0.004 | 73 ± 5 |
| 1.5:5 | 39 ± 1.7 | 0.46 ± 0.04 | 45 ± 0.9 | 0.40 ± 0.009 | 69 ± 4 |
| 2:5 | 57 ± 0.8 | 0.50 ± 0.02 | 63 ± 1.9 | 0.46 ± 0.010 | 66 ± 4 |
| DNA Concentration (μg/mL) | Z-Average Size (nm) | Polydispersity Index (PDI) | Z—Average Size after 72 h (nm) | (PDI) after 72 h | Encapsulation Efficiency (%) |
|---|---|---|---|---|---|
| 15 ± 5 | 40 ± 0.8 | 0.59 ± 0.01 | 50 ± 4.8 | 0.39 ± 0.26 | 56 ± 28 |
| 25 ± 5 | 35 ± 0.5 | 0.48 ± 0.05 | 35 ± 0.5 | 0.53 ± 0.006 | 60 ± 13 |
| 35 ± 5 | 36 ± 0.8 | 0.47 ± 0.05 | 35 ± 0.3 | 0.56 ± 0.006 | 59 ± 4 |
| 45 ± 5 | 38 ± 0.7 | 0.47 ± 0.04 | 36 ± 0.4 | 0.55 ± 0.01 | 69 ± 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nunes, R.; Sousa, Â.; Simaite, A.; Aido, A.; Buzgo, M. Sub-100 nm Chitosan-Triphosphate-DNA Nanoparticles for Delivery of DNA Vaccines. Proceedings 2021, 78, 12. https://doi.org/10.3390/IECP2020-08653
Nunes R, Sousa Â, Simaite A, Aido A, Buzgo M. Sub-100 nm Chitosan-Triphosphate-DNA Nanoparticles for Delivery of DNA Vaccines. Proceedings. 2021; 78(1):12. https://doi.org/10.3390/IECP2020-08653
Chicago/Turabian StyleNunes, Renato, Ângela Sousa, Aiva Simaite, Ahmed Aido, and Matej Buzgo. 2021. "Sub-100 nm Chitosan-Triphosphate-DNA Nanoparticles for Delivery of DNA Vaccines" Proceedings 78, no. 1: 12. https://doi.org/10.3390/IECP2020-08653
APA StyleNunes, R., Sousa, Â., Simaite, A., Aido, A., & Buzgo, M. (2021). Sub-100 nm Chitosan-Triphosphate-DNA Nanoparticles for Delivery of DNA Vaccines. Proceedings, 78(1), 12. https://doi.org/10.3390/IECP2020-08653







