Pharmacokinetic Appraisal of Carprofen Delivery from Intra-Articular Nanoparticles: A Population Modeling Approach in Rabbits †
Abstract
:1. Introduction
2. Experiments
2.1. Reagents and Substances and Assay Solutions
2.2. Animals and Drug Administration
2.3. Sampling Procedures
2.4. Analysis of CP in Plasma and Knee Samples
2.5. Data Analysis. Non-Compartmental/Compartmental Pharmacokinetic/Deconvolution Analysis
3. Results
3.1. Carprofen Concentrations
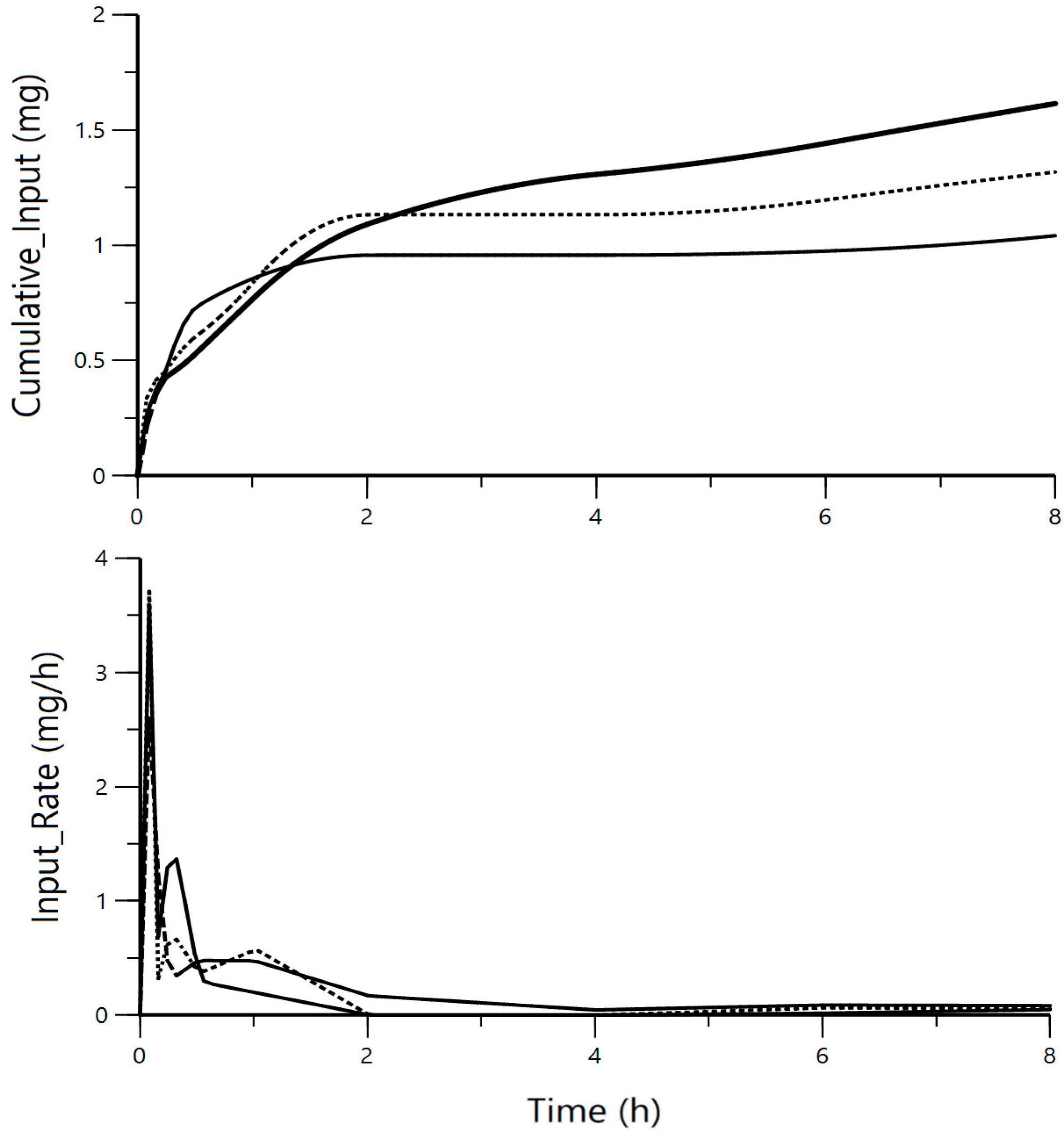
3.2. Pharmacokinetic Analysis
4. Discussion
5. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CP | Carprofen |
| CLD | Distributional clearance |
| IA | Intraarterial |
| NSAID | Non-steroidal anti-inflammatory drug |
References
- Lees, P.; Aliabadi, F.S.; Landoni, M.F. Pharmacodynamics and Enantioselective Pharmacokinetics of Racemic Carprofen in the Horse. J. Vet. Pharmacol. Ther. 2002, 25, 433–448. [Google Scholar] [CrossRef] [PubMed]
- Lipscomb, V.; AliAbadi, F.; Lees, P.; Pead, M.; Muir, P. Clinical Efficacy and Pharmacokinetics of Carprofen in the Treatment of Dogs with Osteoarthritis. Vet. Rec. 2002, 22, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Skjodt, N.; Davies, N. Clinical Pharmacokinetics and Pharmacodynamics of Bromfenac. Clin. Pharmacokinet. 1999, 36, 399–408. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G. Intra-Articular Lornoxicam Loaded PLGA Microspheres: Enhanced Therapeutic Efficiency and Decreased Systemic Toxicity in the Treatment of Osteoarthritis. Drug Deliv. 2012, 19, 255–263. [Google Scholar] [CrossRef]
- Edwards, S.H.R. Intra-Articular Drug Delivery: The Challenge to Extend Drug Residence Time within the Joint. Vet. J. 2011, 190, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Tunçay, M.; Çaliş, S.; Kaş, H.S.; Ercan, M.T.; Peksoy, I.; Hincal, A.A. Diclofenac Sodium Incorporated PLGA (50:50) Microspheres: Formulation Considerations and in Vitro/in Vivo Evaluation. Int. J. Pharm. 2000, 195, 179–188. [Google Scholar] [CrossRef]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (Part 1): Products on the Market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef]
- Jiang, D.; Zou, J.; Huang, L.; Shi, Q.; Zhu, X.; Wang, G.; Yang, H. Efficacy of Intra-Articular Injection of Celecoxib in a Rabbit Model of Osteoarthritis. Int. J. Mol. Sci. 2010, 11, 4106–4113. [Google Scholar] [CrossRef]
- Horisawa, E.; Kubota, K.; Tuboi, I.; Sato, K.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Size-Dependency of DL-Lactide/Glycolide Copolymer Particulates for Intra-Articular Delivery System on Phagocytosis in Rat Synovium. Pharm. Res. 2002, 19, 132–139. [Google Scholar] [CrossRef]
- Horisawa, E.; Hirota, T.; Kawazoe, S.; Yamada, J.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Prolonged Anti-Inflammatory Action of DL-Lactide/Glycolide Copolymer Nanospheres Containing Betamethasone Sodium Phosphate for an Intra-Articular Delivery System in Antigen-Induced Arthritic Rabbit. Pharm. Res. 2002, 19, 403–410. [Google Scholar] [CrossRef]
- Lentner, C. Geigy Scientific Tables, 8th ed.; Journal of the Royal Society of Medicine: Basel, Switzerland, 1981. [Google Scholar]
- Parton, K.; Balmer, T.V.; Boyle, J.; Whittem, T.; Machon, R. The Pharmacokinetics and Effects of Intravenously Administered Carprofen and Salicylate on Gastrointestinal Mucose and Selected Biochemical Measurements in Healthy Cats. J. Vet. Pharmacol. Ther. 2000, 23, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Parra, A.; Clares, B.; Rosselló, A.; Garduño-Ramírez, M.L.; Abrego, G.; García, M.L.; Calpena, A.C. Ex Vivo Permeation of Carprofen from Nanoparticles: A Comprehensive Study through Human, Porcine and Bovine Skin as Anti-Inflammatory Agent. Int. J. Pharm. 2016, 501, 10–17. [Google Scholar] [CrossRef]
- Gibaldi, M.; Perrier, D. Farmacocinética; Reverté: Barcelona, Spain, 1982. [Google Scholar]
- Veng-Pedersen, P. Linear and Nonlinear System Approaches in Pharmacokinetics: How Much Do They Have to Offer? I General Considerations. J. Pharmacokinet. Biopharm. 1988, 16, 413–472. [Google Scholar] [CrossRef] [PubMed]
- Bauer, R. Nonmem Users Guide Introduction to Nonmem 7.2.0. J. Chem. Inf. Model. 2011, 53, 1689–1699. [Google Scholar]
- Hooker, A.C.; Staatz, C.E.; Karlsson, M.O. Conditional Weighted Residuals (CWRES): A Model Diagnostic for the FOCE Method. Pharm. Res. 2007, 24, 2187–2197. [Google Scholar] [CrossRef]
- Yamaoka, T.; Nakagawa, T.; Uno, T. Application of Akaike’s Information Criterion (AIC) in the Evaluation of Linear Pharmacokinetics Equations. J. Pharmacokinet. Biopharm. 1978, 6, 165–175. [Google Scholar] [CrossRef]
- Holford, N. The Visual Predictive Check Superiority to Standard Diagnostic (Rorschach) Plots. In Proceedings of the PAGE 2005, Pamplona, Spain, 16–17 June 2005; Population Approach Group Europe (PAGE): London, UK. [Google Scholar]
- Anderson, B.J.; Holford, N.H.G. Mechanism-Based Concepts of Size and Maturity in Pharmacokinetics. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 303–332. [Google Scholar] [CrossRef]
- Anderson, B.J.; Holford, N.H.G. Mechanistic Basis of Using Body Size and Maturation to Predict Clearance in Humans. Drug Metab. Pharmacokinet. 2009, 24, 25–36. [Google Scholar] [CrossRef]
- Mahmood, I. Misconceptions and Issues Regarding Allometric Scaling during the Drug Development Process. Expert Opin. Drug Metab. Toxicol. 2018, 14, 843–854. [Google Scholar] [CrossRef]
- Bergstrand, M.; Hooker, A.C.; Wallin, J.E.; Karlsson, M.O. Prediction-Corrected Visual Predictive Checks for Diagnosing Nonlinear Mixed-Effects Models. AAPS J. 2011, 13, 143–151. [Google Scholar] [CrossRef]
- Messenger, K.M.; Wofford, J.A.; Papich, M.G. Carprofen Pharmacokinetics in Plasma and in Control and Inflamed Canine Tissue Fluid Using in vivo Ultrafiltration. J. Vet. Pharmacol. Ther. 2016, 39, 32–39. [Google Scholar] [CrossRef]
- Sidler, M.; Fouché, N.; Meth, I.; von Hahn, F.; von Rechenberg, B.; Kronen, P.W. Preliminary Study on Carprofen Concentration Measurements after Transcutaneous Treatment with Vetdrop® in a Microfracture Joint Defect Model in Sheep. BMC Vet. Res. 2014, 10, 268. [Google Scholar] [CrossRef]
- Hawkins, M.G.; Taylor, I.T.; Craigmill, A.L.; Tell, L.A. Enantioselective Pharmacokinetics of Racemic Carprofen in New Zealand White Rabbits. J. Vet. Pharmacol. Ther. 2008, 31, 423–430. [Google Scholar] [CrossRef]
- Taylor, P.M.; Delatour, P.; Landoni, F.M.; Deal, C.; Pickett, C.; Aliabadi, R.S.; Foot, P.; Lees, P. Pharmacodynamics and Enantioselective Pharmacokinetics of Carprofen in the Cat. Res. Vet. Sci. 1996, 60, 144–151. [Google Scholar] [CrossRef]
- Panaretto, B.A. Body Composition In Vivo. I. The Estimation of Total Body Water with Antipyrine and the Relation of Total Body Water to Total Body Fat in Rabbits. Aust. J. Agric. Res. 1963, 14, 594–601. [Google Scholar] [CrossRef]
- Crevoisier, C. Pharmacokinetic Properties of Carprofen in Humans. Eur. J. Rheumatol. Inflamm. 1982, 5, 492–502. [Google Scholar] [PubMed]
- Ray, J.E.; Wade, D. The Pharmacokinetics and Metabolism of 14C-Carprofen in Man. Biopharm. Drug Dispos. 1982, 3, 29–38. [Google Scholar] [CrossRef]
- Morille, M.; Van-Thanh, T.; Garric, X.; Cayon, J.; Coudane, J.; Noël, D.; Venier-Julienne, M.C.; Montero-Menei, C.N. New PLGA-P188-PLGA Matrix Enhances TGF-Β3 Release from Pharmacologically Active Microcarriers and Promotes Chondrogenesis of Mesenchymal Stem Cells. J. Control. Release 2013, 170, 99–110. [Google Scholar] [CrossRef]
- Miciletta, M.; Cuniberti, B.; Barbero, R.; Re, G. In Vitro Enantioselective Pharmacodynamics of Carprofen and Flunixin-Meglumine in Feedlot Cattle. J. Vet. Pharmacol. Ther. 2014, 37, 43–52. [Google Scholar] [CrossRef]
- Parra, A.; Mallandrich, M.; Clares, B.; Egea, M.A.; Espina, M.; García, M.L.; Calpena, A.C. Design and Elaboration of Freeze-Dried PLGA Nanoparticles for the Transcorneal Permeation of Carprofen: Ocular Anti-Inflammatory Applications. Colloids Surfaces B Biointerfaces 2015, 136, 935–943. [Google Scholar] [CrossRef]
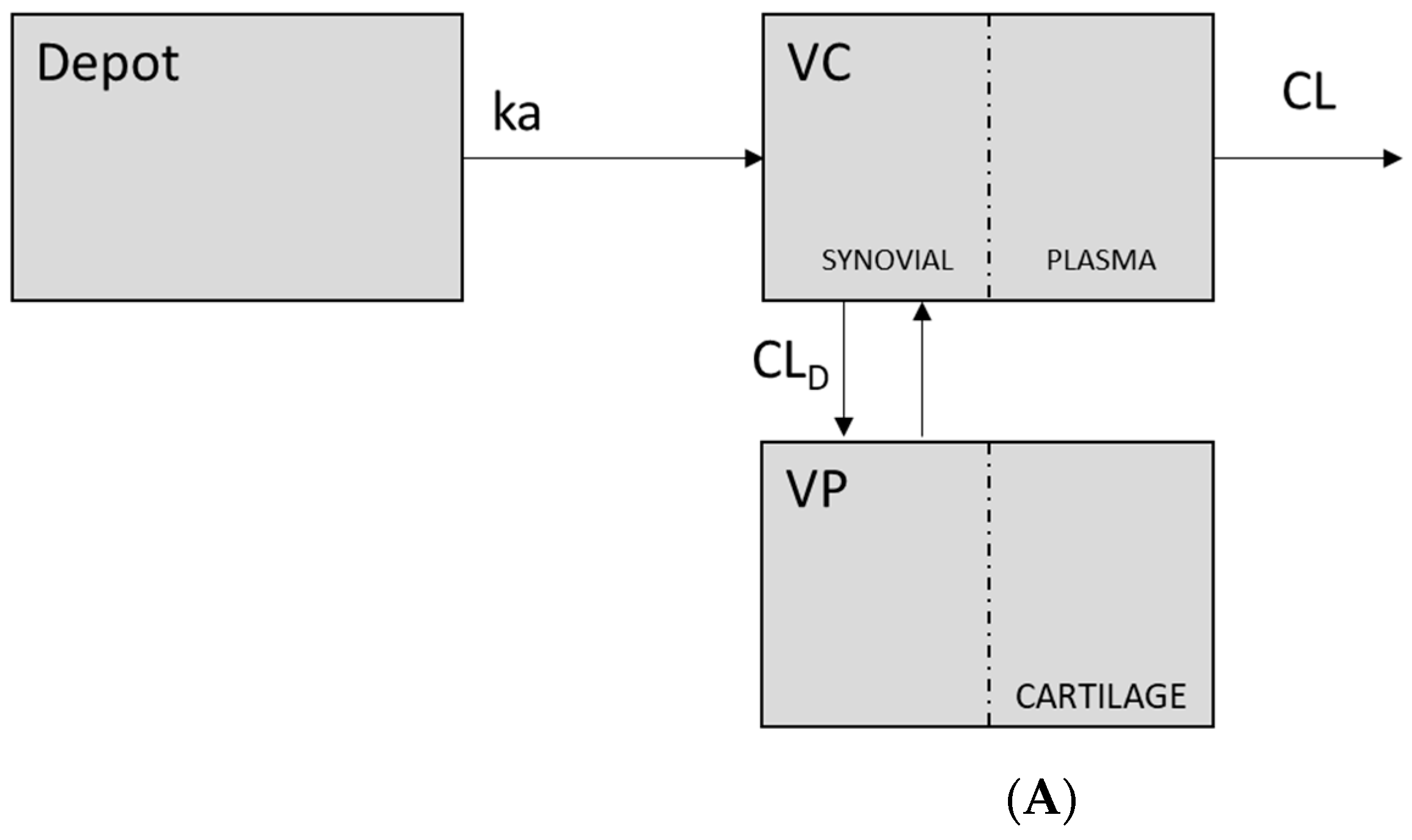
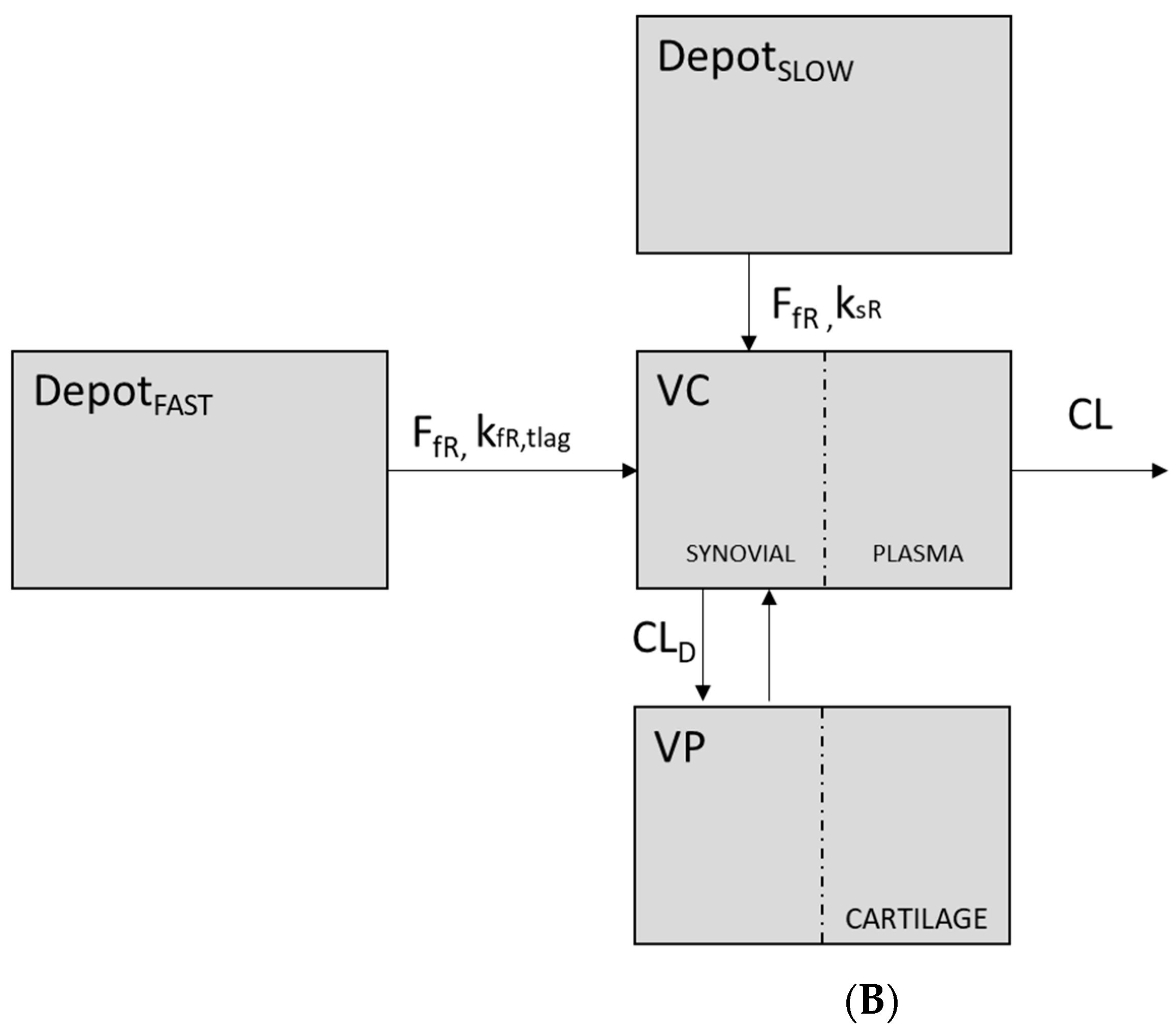


| Model | Kinetics of the Release/Absorption Process | Sequential vs. Parallel | Number of Depots |
|---|---|---|---|
| 1 | 1st order absorption (ka) | - | one |
| 2 | Zero order absorption (ka) | - | one |
| 3 | Two 1st order absorption processes (kfr, ksr) | Parallel | two |
| 4 | 1st order (kfr) and zero order absorption processes (ksr) | Parallel | two |
| 5 | 1st order (kfr) and zero order absorption processes (ksr) | Sequential | two |
| 6 | Two 1st order absorption processes (kfr, ksr) | Sequential | two |
| Tissue | Concentration (µg/g) * |
|---|---|
| Cartilage | 0.997 |
| Meniscus | 0.099 |
| Synovial fluid | 0.049 |
| Plasma | 0.3 |
| Parameter | Intravenous Administration | Intra-Articular Administration |
|---|---|---|
| λz (h−1) | 0.3565 ± 0.1546 | 0.1892 ± 0.0436 |
| t1/2λz (h) | 2.16 ± 0.76 | 3.78 ± 0.78 |
| AUC (mg/L)·h | 65.03 ± 20.90 | 6.73 ± 0.38 |
| AUC/D | 4.24 ± 1.36 | 3.40 ± 0.19 |
| AUCextrap (%) | 4.18 ± 4.06 | 28.29 ± 2.92 |
| CL (L/h) | 0.2533 ± 0.0831 | - |
| Vi (L) | 0.2058 ± 0.0273 | - |
| Vss (L) | 0.4403 ± 0.0758 | - |
| Vdarea (L) | 0.7963 ± 0.414 | - |
| Cmax (mg/L) | 75.67 ± 12.40 | 1.84 ± 0.19 |
| Cmax/D | 4.93 ± 0.81 | 0.93 ± 0.96 |
| Tmax (h) | - | 0.25 (0.08–0.5) |
| F (%) | - | 94.48 ± 27.83 |
| Parameter | Units | Final Model Parameter Estimate (RSE%) |
|---|---|---|
| Disposition parameters | ||
| CL | L/h·70 kg | 2.00 (8.25) |
| VC | L/70 kg | 3.60 (9.36) |
| CLD | L/h·70 kg | 3.08 (4.90) |
| VP | L/70 kg | 4.28 (14.21) |
| Release/Absorption parameters | ||
| F | % | 51.4 (9.82) |
| KaFR | h−1 | 7.38 (9.17) |
| KaSR | h−1 | 0.0667 (15.59) |
| FFR | % | 0.595 (1.20) |
| FSR | % | 0.405 |
| TlagSR | h | 0.5 (0.11) |
| Inter-individual variability | ||
| IIVCL | % | 19.21 (62.6) |
| Residual variability | ||
| Proportional | % | 25.51 (29.34) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parra-Coca, A.; Boix-Montañés, A.; Calpena, A.C.; Colom, H. Pharmacokinetic Appraisal of Carprofen Delivery from Intra-Articular Nanoparticles: A Population Modeling Approach in Rabbits. Proceedings 2021, 78, 11. https://doi.org/10.3390/IECP2020-08677
Parra-Coca A, Boix-Montañés A, Calpena AC, Colom H. Pharmacokinetic Appraisal of Carprofen Delivery from Intra-Articular Nanoparticles: A Population Modeling Approach in Rabbits. Proceedings. 2021; 78(1):11. https://doi.org/10.3390/IECP2020-08677
Chicago/Turabian StyleParra-Coca, Alexander, Antonio Boix-Montañés, Ana C. Calpena, and Helena Colom. 2021. "Pharmacokinetic Appraisal of Carprofen Delivery from Intra-Articular Nanoparticles: A Population Modeling Approach in Rabbits" Proceedings 78, no. 1: 11. https://doi.org/10.3390/IECP2020-08677
APA StyleParra-Coca, A., Boix-Montañés, A., Calpena, A. C., & Colom, H. (2021). Pharmacokinetic Appraisal of Carprofen Delivery from Intra-Articular Nanoparticles: A Population Modeling Approach in Rabbits. Proceedings, 78(1), 11. https://doi.org/10.3390/IECP2020-08677







