Cytomorphological, Molecular Diagnosis and Evaluation of Insertion of the LINE-1 Element in the C-MYC Gene in Canine Transmissible Venereal Tumor: Applicability in Veterinary Clinical Routine †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Aspects of the Research
2.2. Sample Collection
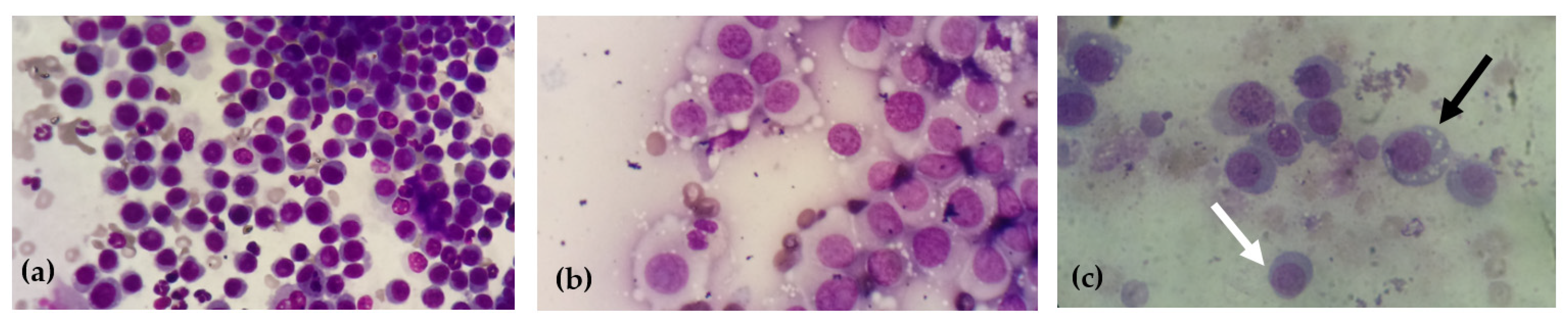
2.3. Cytological Diagnosis and Cytomorphological Classification
2.4. DNA Extraction, Amplification, Sequencing, and Alignment
3. Results and Discussion
4. Conclusions
Institutional Review Board Statement
Acknowledgments
References
- Goldschmidt, M.H.; Hendrick, M.J. Tumors of the skin and soft tissues. In Tumors in Domestic Animals, 4th ed.; Meuten, D.J., Ed.; Iowa State: Ames, IA, USA, 2002; Chapter 2; pp. 45–118. [Google Scholar]
- Ostrander, E.A.; Davis, B.W.; Ostrander, G.K. Transmissible Tumors: Breaking the Cancer Paradigm. Trends Genet. 2016, 32, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Murgia, C.; Pritchard, J.K.; Kim, S.Y.; Fassati, A.; Weiss, R.A. Clonal Origin and Evolution of a Transmissible Cancer. Cell 2006, 126, 477–487. [Google Scholar] [CrossRef] [PubMed]
- Rebbeck, C.A.; Thomas, R.; Breen, M.; Leroi, A.M.; Burt, A. Origins and evolution of a transmissible cancer. Evolution 2009, 63, 2340–2349. [Google Scholar] [CrossRef] [PubMed]
- Setthawongsin, C.; Tangkawattana, S.; Rungsipipat, A.; Techangamsuwan, S. Computerized Cytomorphometric and Cytomorphological Analysis of Canine Transmissible Venereal Tumours. J. Comp. Pathol. 2018, 163, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Strakova, A.; Murchison, E.P. The cancer which survived: Insights from the genome of an 11,000 year-old cancer. Curr. Opin. Genet. 2015, 30, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Park, M.S.; Kim, Y.; Kang, M.S.; Oh, S.Y.; Cho, D.Y.; Shin, N.S.; Kim, D.Y. Disseminated transmissible venereal tumor in a dog. J. Vet. Diagn. Investig. 2006, 18, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Das, U.; Das, A.K. Review of canine transmissible venereal sarcoma. Vet. Res. Commun. 2000, 24, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, L.S.; Mota, L.S.; Colodel, M.M.; Ferreira, I.; Brandão, C.V.S.; Rocha, N.S. Spontaneous canine transmissible venereal tumor: Association between different phenotypes and the insertion LINE-1/c-myc. Rev. Colomb. Cienc. Pecu. 2012, 25, 402–408. [Google Scholar]
- Amaral, A.S.; Bassani-Silva, S.; Ferreira, I.; Fonseca, L.S.; Andrade, F.H.E.; Gaspar, L.F.J.; Rocha, N.S. Cytomorphological characterization of transmissible canine venereal tumor. Rev. Port. Cienc. Vet. 2007, 102, 253–260. [Google Scholar]
- Florez, M.M.; Feo, H.; Yamatogi, R.; Yamatogi, R.S.; Aguiar, A.J.; Araújo, J.P., Jr.; Rocha, N.S. Cell cycle kinetics, apoptosis rates and gene expressions of MDR-1, TP53, Bcl-2 and BAX in transmissible venereal tumor cells and their association with therapy response. Vet. Comparat. Oncol. 2016, 14, 1–15. [Google Scholar]
- Lima, C.R.O.; Faleiro, M.B.R.; Rabelo, R.E.; Vulcani, V.A.S.; Rubini, M.R.; Torres, F.A.G.; Moura, V.M.B.D. Insertion of the LINE-1 element in the C-MYC gene and immunoreactivity of C-MYC, p53, p21 and p27 proteins in different morphological patterns of the canine TVT. Arq. Bras. Med. Vet. Zootec. 2016, 68, 658–666. [Google Scholar] [CrossRef]
- Flórez, L.M.M. Expressão dos Genes MDR-1, TP53, BCL-2 E BAX em Tumor Venéreo Transmissível Canino e sua Relação com a Agressividade e Resposta à Terapia. Ph.D. Thesis, Unesp, Botucatu, 2014; p. 81. [Google Scholar]
- Spin, J.S.F.; Da Mota, L.S.; DA Castelli, L.S.L.S.; Silva, E.C.; Ferreira, S.B.; Rocha, I.; Sousa, N. Detecção molecular do rearranjo Line-1/c-MYC em tumores venéreos transmissíveis caninos espontâneos. Clín. Vet. 2010, 15, 64–68. [Google Scholar]
- Silva, J.S.; Da Silva, D.M.M.; Silva, V.B.; Da Silva, D.A.; Silva, R.S.; De Lucena, R.B. Classificação citopatológica e epidemiologia do TVT nos cães atendidos no Hospital Veterinário da UFPB. In Proceedings of the 38º Congresso Brasileiro Da Anclivepa 2017, Anais do 38º CBA, Recife, Brazil, 2017; p. 0808. [Google Scholar]
- Gaspar, L.F.; Ferreira, I.; Colodel, M.M.; Brandão, C.V.; Rocha, N.S. Spontaneus canine transmissible venereal tumor: Cell morphology and influence on p-glycoprotein expression. Turk. J. Vet. Anim. Sci. 2010, 34, 447–454. [Google Scholar]
- Valençola, R.A.; Antunes, T.R.; Sorgatto, S.; Oliveira, B.B.; Godoy, K.C.S.; De Souza, A.I. Aspectos citomorfológicos e frequência dos subtipos do tumor venéreo transmissível canino no município de Campo Grande, Mato Grosso do Sul, Brasil. Acta Vet. Bras. 2015, 9, 82–86. [Google Scholar]
- O’neill, I.D. Concise review: Transmissible animal tumors as models of the cancer stem-cell process. Cancer Stem. Cells 2011, 29, 1909–1914. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, F.T.A.; Silva, F.W.A.; Correa, D.O.E.H. Cytomorphological, Molecular Diagnosis and Evaluation of Insertion of the LINE-1 Element in the C-MYC Gene in Canine Transmissible Venereal Tumor: Applicability in Veterinary Clinical Routine. Proceedings 2021, 76, 4. https://doi.org/10.3390/IECGE-07156
Silva FTA, Silva FWA, Correa DOEH. Cytomorphological, Molecular Diagnosis and Evaluation of Insertion of the LINE-1 Element in the C-MYC Gene in Canine Transmissible Venereal Tumor: Applicability in Veterinary Clinical Routine. Proceedings. 2021; 76(1):4. https://doi.org/10.3390/IECGE-07156
Chicago/Turabian StyleSilva, Faro Thamirys Aline, Ferreira Wallax Augusto Silva, and De Oliveira Edivaldo Herculano Correa. 2021. "Cytomorphological, Molecular Diagnosis and Evaluation of Insertion of the LINE-1 Element in the C-MYC Gene in Canine Transmissible Venereal Tumor: Applicability in Veterinary Clinical Routine" Proceedings 76, no. 1: 4. https://doi.org/10.3390/IECGE-07156
APA StyleSilva, F. T. A., Silva, F. W. A., & Correa, D. O. E. H. (2021). Cytomorphological, Molecular Diagnosis and Evaluation of Insertion of the LINE-1 Element in the C-MYC Gene in Canine Transmissible Venereal Tumor: Applicability in Veterinary Clinical Routine. Proceedings, 76(1), 4. https://doi.org/10.3390/IECGE-07156




