Fat Fraction Qualitative Characteristics for Oat-Based Products †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Extraction of Fat from Oat-based Products
2.2.2. Gas Chromatography
2.2.3. Enzymatic Hydrolysis of Triacylglycerols
2.2.4. Pressure Differential Scanning Calorimetry (PDSC)
2.2.5. Differential Scanning Calorimetry
2.2.6. Statistical Analysis
3. Results
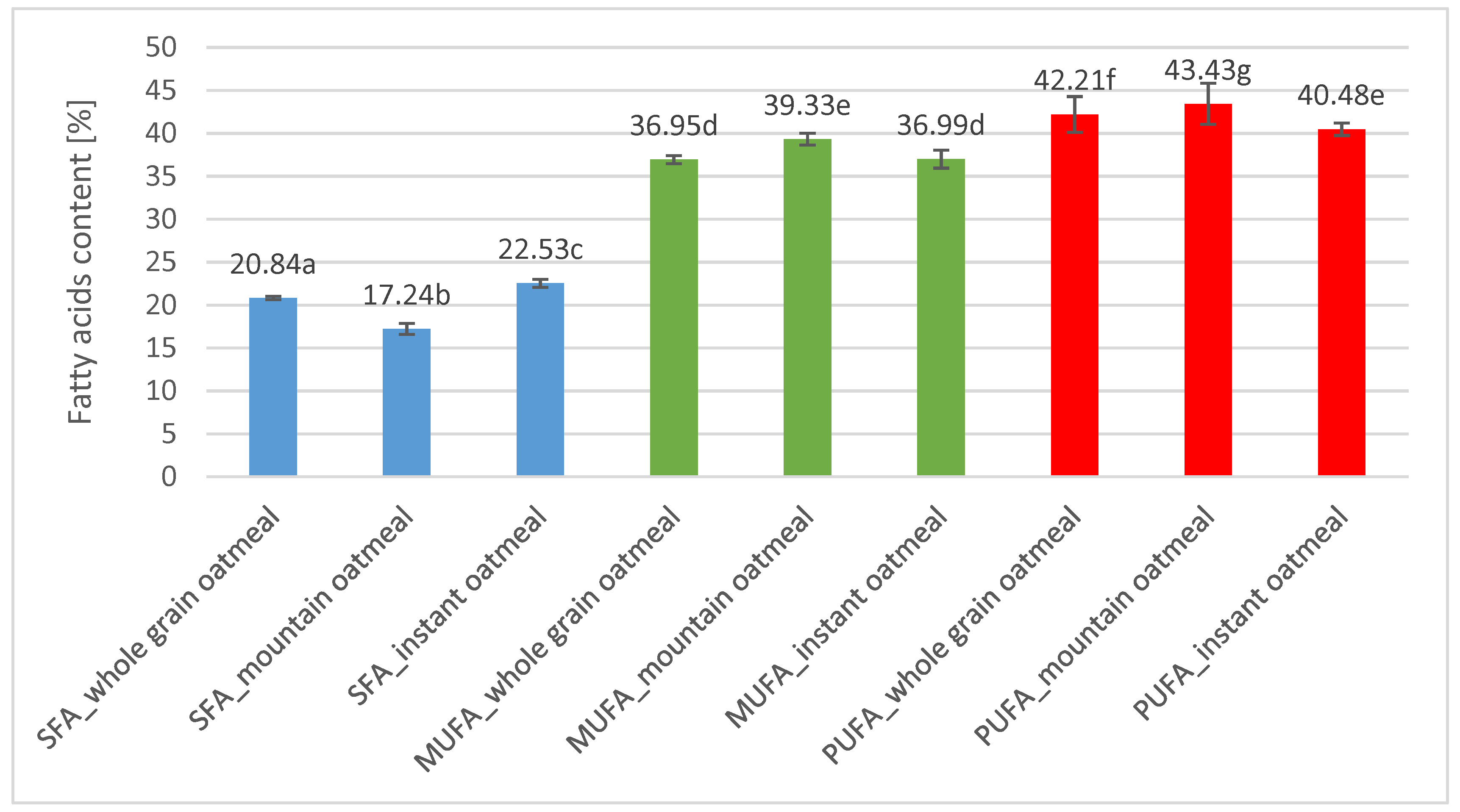
3.1. Fatty Acids Composition and Distribution in Triacylglycerols
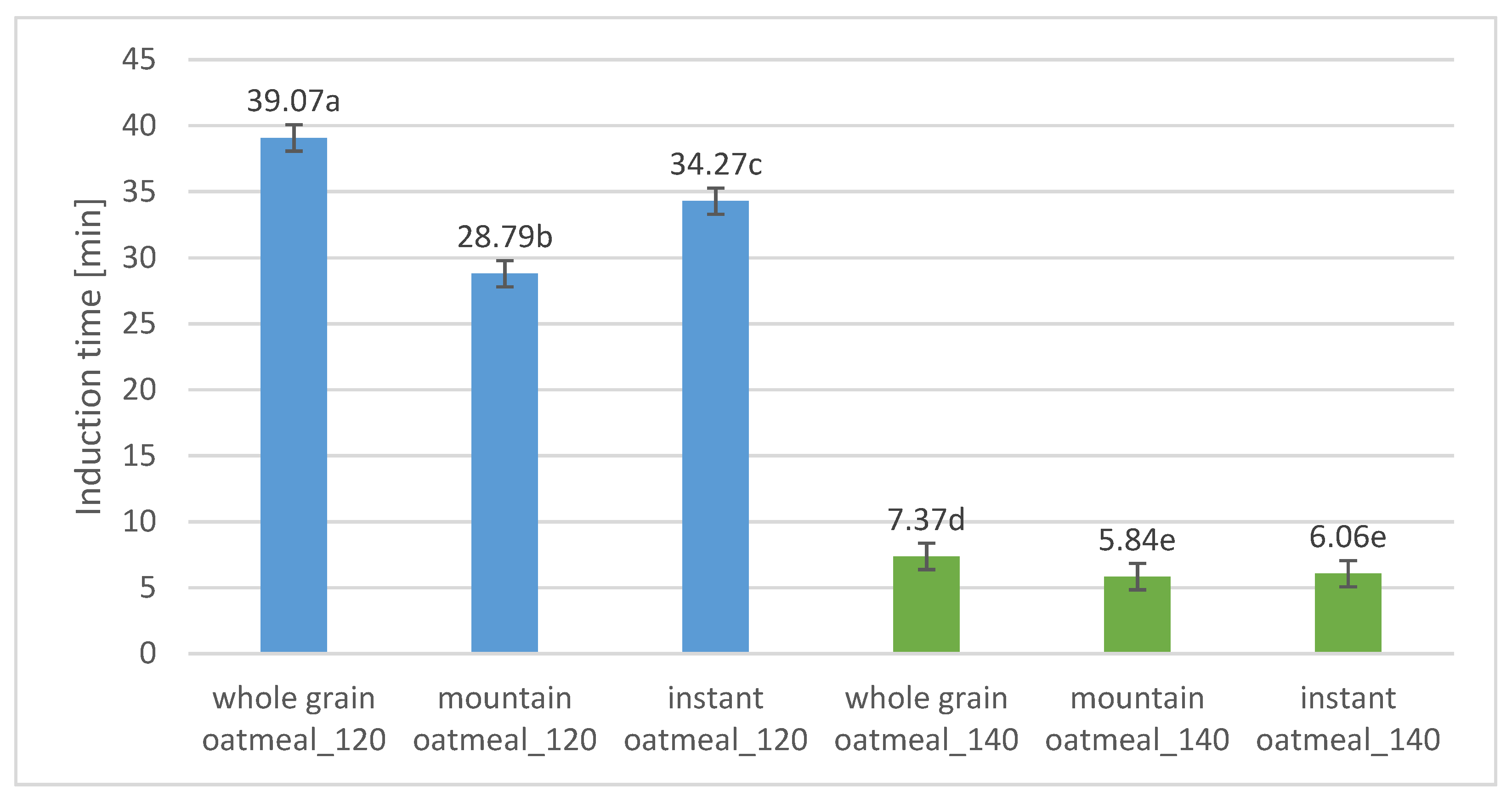
3.2. Oxidative Stability of Fats
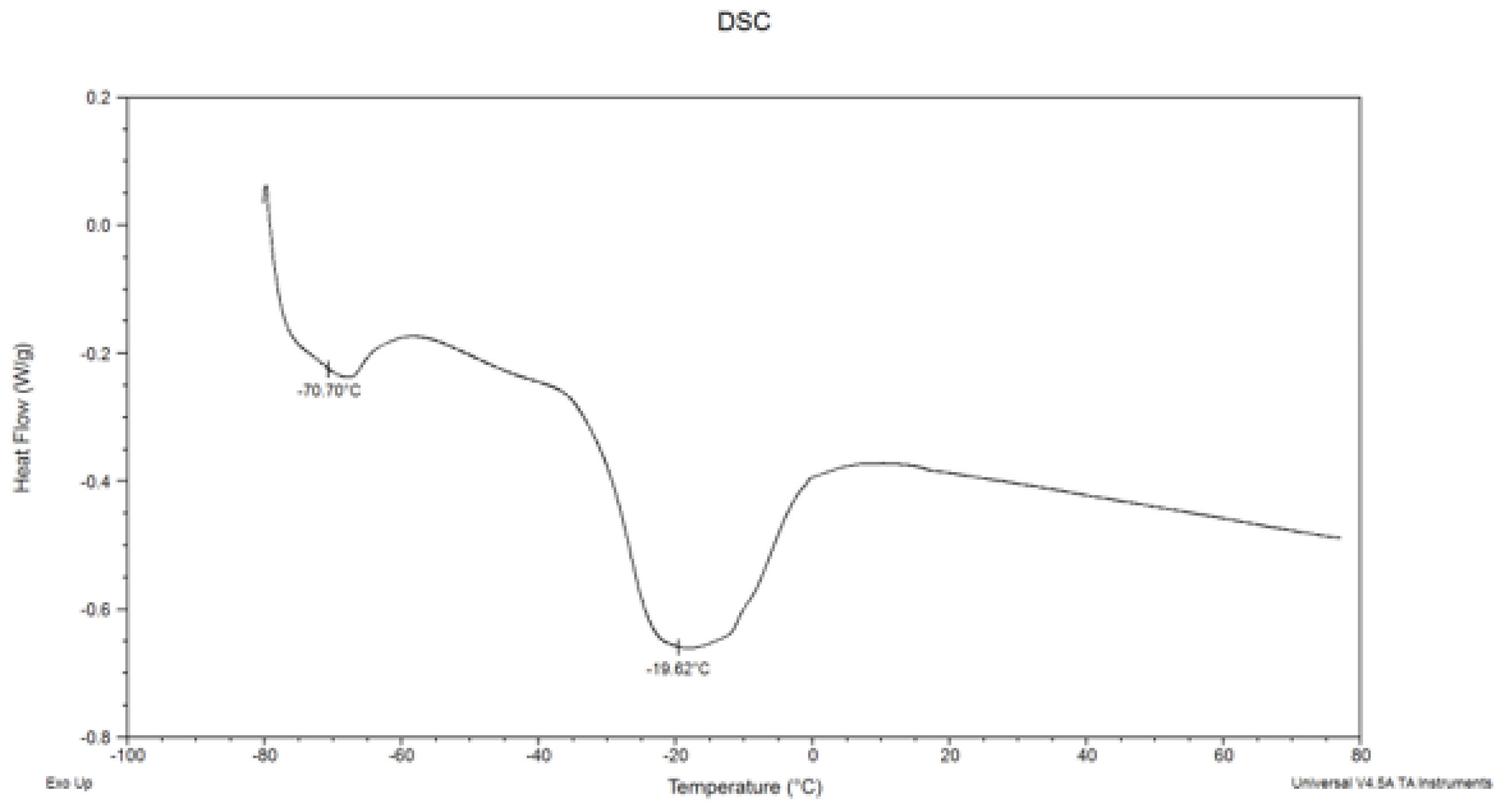
3.3. Melting Characteristics of Fat
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Butt, M.S.; Tahir-Nadeem, M.; Khan, M.K.I.; Shabir, R.; Butt, M.S. Oat: Unique among the cereals. Eur. J. Nutr. 2008, 47, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Lásztity, R. Oat grain—A wonderful reservoir of natural nutrients and biologically active substances. Food Rev. Int. 1998, 14, 99–119. [Google Scholar] [CrossRef]
- Sangwan, S.; Singh, R.; Tomar, S.K. Nutritional and functional properties of oats: An update. J. Innov. Biol. 2014, 1, 3–14. [Google Scholar]
- Rasane, P.; Jha, A.; Sabikhi, L.; Kumar, A.; Unnikrishnan, V.S. Nutritional advantages of oats and opportunities for its processing as value added foods—A review. J. Food Sci. Technol. 2015, 52, 662–675. [Google Scholar] [CrossRef] [PubMed]
- Reder, M.; Koczoń, P.; Wirkowska-Wojdyła, M.; Sujka, K.; Ciemniewska-Żytkiewicz, H. The Application of FT-MIR Spectroscopy for the Evaluation of Energy Value, Fat Content, and Fatty Acid Composition in Selected Organic Oat Products. Food Anal. Methods 2014, 7, 547–554. [Google Scholar] [CrossRef]
- Lange, E. Oat products as a functional food. ŻNTJ 2010, 17, 7–24. [Google Scholar]
- Arcos, J.A.; Garcia, H.; Hill, C.G. Regioselective analysis of the fatty acid composition of triacylglycerols with conventional high-performance liquid chromatography. J. Am. Oil Chem. Soc. 2000, 77, 507–512. [Google Scholar] [CrossRef]
- Hunter, J.E. Studies on effects of dietary fatty acids as related to their position on triglycerides. Lipids 2001, 36, 655–668. [Google Scholar] [CrossRef]
- Kowalski, B.; Gruczyńska, E.; Maciaszek, K. Kinetics of rapeseed oil oxidation by pressure differential scanning calorimetry measurements. Eur. J. Lipid Sci. Technol. 2000, 102, 337–341. [Google Scholar] [CrossRef]
- Ciemniewska-Żytkiewicz, H.; Ratusz, K.; Bryś, J.; Reder, M.; Koczoń, P. Determination of the oxidative stability of hazelnut oils by PDSC and Rancimat methods. J. Therm. Anal. Calorim. 2014, 118, 875–881. [Google Scholar] [CrossRef]
- Knothe, G.; Dunn, R.O. A Comprehensive Evaluation of the Melting Points of Fatty Acids and Esters Determined by Differential Scanning Calorimetry. J. Am. Oil Chem. Soc. 2009, 86, 843–856. [Google Scholar] [CrossRef]
| Fatty Acid | Fatty Acid Composition in TAG (%) | Fatty Acid Composition in Positions | Fatty Acid Share in sn-2 Position (%) | |
|---|---|---|---|---|
| sn-2 | sn-1,3 | |||
| C 16:0 | 18.02 ± 1.09 | 3.41 ± 0.7 | 25.33 ± 2.44 | 6.30 ± 1.15 |
| C 18:0 | 1.87 ± 0.18 | 0.51 ± 0.09 | 2.56 ± 0.53 | 9.00 ± 0.85 |
| C 18:1 n-9 | 35.12 ± 2.75 | 48.59 ± 2.21 | 28.39 ± 1.97 | 46.12 ± 2.45 |
| C 18:2 n-6 | 39.83 ± 1.54 | 44.73 ± 3.11 | 37.39 ± 1.65 | 37.44 ± 1.63 |
| C 18:3 n-3 | 1.71 ± 0.28 | 1.66 ± 0.14 | 1.73 ± 0.23 | 32.36 ± 2.04 |
| Fatty Acid | Fatty Acid Composition in TAG (%) | Fatty Acid Composition in Positions | Fatty Acid Share in sn-2 Position (%) | |
|---|---|---|---|---|
| sn-2 | sn-1,3 | |||
| C 16:0 | 16.13 ± 1.45 | 4.14 ± 0.18 | 22.12 ± 1.74 | 8.56 ± 1.48 |
| C 18:0 | 1.28 ± 0.07 | 0.67 ± 0.07 | 1.59 ± 0.12 | 17.33 ± 1.37 |
| C 18:1 n-9 | 37.57 ± 2.05 | 48.28 ± 2.78 | 32.23 ± 2.06 | 42.83 ± 3.71 |
| C 18:2 n-6 | 41.57 ± 3.14 | 44.08 ± 1.93 | 40.32 ± 3.07 | 35.35 ± 2.85 |
| C 18:3 n-3 | 0.61 ± 0.08 | 1.57 ± 0.04 | 0.13 ± 0.05 | 35.51 ± 2.43 |
| Fatty Acid | Fatty Acid Composition in TAG (%) | Fatty Acid Composition in Positions | Fatty Acid Share in sn-2 Position (%) | |
|---|---|---|---|---|
| sn-2 | sn-1,3 | |||
| C 16:0 | 18.81 ± 1.75 | 4.45 ± 0.86 | 25.99 ± 2.64 | 7.88 ± 0.83 |
| C 18:0 | 2.27 ± 0.04 | 0.81 ± 0.06 | 3.00 ± 0.06 | 11.85 ± 1.95 |
| C 18:1 n-9 | 35.32 ± 2.04 | 48.11 ± 2.14 | 28.93 ± 1.63 | 45.41 ± 2.38 |
| C 18:2 n-6 | 38.35 ± 2.14 | 43.64 ± 2.85 | 35.71 ± 2.36 | 37.94 ± 2.15 |
| C 18:3 n-3 | 1.49 ± 0.05 | 1.46 ± 0.07 | 1.51 ± 0.08 | 32.66 ± 1.97 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górska, A.; Brzezińska, R.; Chojnacka, K.; Bryś, J.; Ostrowska-Ligęza, E.; Wirkowska-Wojdyła, M.; Dolatowska-Żebrowska, K. Fat Fraction Qualitative Characteristics for Oat-Based Products. Proceedings 2021, 70, 93. https://doi.org/10.3390/foods_2020-07707
Górska A, Brzezińska R, Chojnacka K, Bryś J, Ostrowska-Ligęza E, Wirkowska-Wojdyła M, Dolatowska-Żebrowska K. Fat Fraction Qualitative Characteristics for Oat-Based Products. Proceedings. 2021; 70(1):93. https://doi.org/10.3390/foods_2020-07707
Chicago/Turabian StyleGórska, Agata, Rita Brzezińska, Klaudia Chojnacka, Joanna Bryś, Ewa Ostrowska-Ligęza, Magdalena Wirkowska-Wojdyła, and Karolina Dolatowska-Żebrowska. 2021. "Fat Fraction Qualitative Characteristics for Oat-Based Products" Proceedings 70, no. 1: 93. https://doi.org/10.3390/foods_2020-07707
APA StyleGórska, A., Brzezińska, R., Chojnacka, K., Bryś, J., Ostrowska-Ligęza, E., Wirkowska-Wojdyła, M., & Dolatowska-Żebrowska, K. (2021). Fat Fraction Qualitative Characteristics for Oat-Based Products. Proceedings, 70(1), 93. https://doi.org/10.3390/foods_2020-07707









