Valorization of Kiwi by-Products for the Recovery of Bioactive Compounds: Circular Economy Model †
Abstract
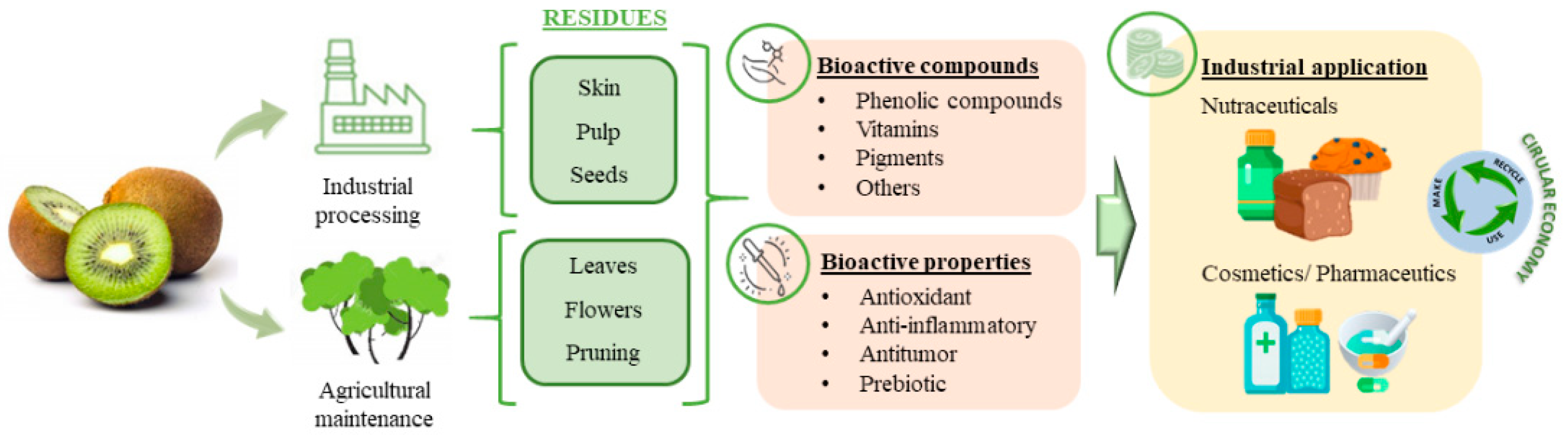
1. Introduction
2. Associated Waste to Kiwi Production
2.1. Waste Derived from the Industrial Production of Kiwi Fruit
2.2. Waste Derived from the Maintenance of Industrial Operations of the Kiwi Plant
3. Biological Activity
4. Sustainable Valorization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Latocha, P. The Nutritional and Health Benefits of Kiwiberry (Actinidia arguta)—A Review. Plant Foods Hum. Nutr. 2017, 72, 325–334. [Google Scholar] [CrossRef]
- Nishiyama, I.; Yamashita, Y.; Yamanaka, M.; Shimohashi, A.; Fukuda, T.; Oota, T. Varietal Difference in Vitamin C Content in the Fruit of Kiwifruit and Other Actinidia Species. J. Agric. Food Chem. 2004, 52, 5472–5475. [Google Scholar] [CrossRef]
- Pinto, D.; Delerue-matos, C.; Rodrigues, F. Bioactivity, phytochemical profile and pro-healthy properties of Actinidia arguta: A review. Food Res. Int. 2020, 136, 109449. [Google Scholar] [CrossRef] [PubMed]
- Giangrieco, I.; Proietti, S.; Moscatello, S.; Tuppo, L.; Battistelli, A.; La Cara, F.; Tamburrini, M.; Famiani, F.; Ciardiello, M.A.; Agro-ambientale, B.; et al. In fluence of Geographical Location of Orchards on Green Kiwifruit Bioactive Components. J. Agric. Food Chem. 2016, 64, 9172–9179. [Google Scholar] [CrossRef] [PubMed]
- Margarida, A.; Pinto, D.; Fernandes, I.; Gonçalves, T.; Costa, H.S.; Freitas, V.; Rodrigues, F.; Oliveira, M.B.P.P. Infusions and decoctions of dehydrated fruits of Actinidia arguta and Actinidia deliciosa: Bioactivity, radical scavenging activity and e ff ects on cells viability. Food Chem. 2019, 289, 625–634. [Google Scholar] [CrossRef]
- Latocha, P.; Łata, B.; Stasiak, A. Phenolics, ascorbate and the antioxidant potential of kiwiberry vs. common kiwifruit: The effect of cultivar and tissue type. J. Funct. Foods 2015, 19, 155–163. [Google Scholar] [CrossRef]
- Nishiyama, I. Fruits of the Actinidia Genus. Adv. Food Nutr. Res. 2007, 52, 293–324. [Google Scholar] [CrossRef]
- Pérez-Burillo, S.; Oliveras, M.J.; Quesada, J.; Rufián-Henares, J.A.; Pastoriza, S. Relationship between composition and bioactivity of persimmon and kiwifruit. Food Res. Int. 2018, 105, 461–472. [Google Scholar] [CrossRef]
- FAOSTAT Statistical Database Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 31 August 2020).
- Richardson, D.P.; Ansell, J.; Drummond, L.N. The nutritional and health attributes of kiwifruit: A review. Eur. J. Nutr. 2018, 57, 2659–2676. [Google Scholar] [CrossRef]
- Jimenez-Lopez, C.; Fraga-Corral, M.; Carpena, M.; García-Oliveira, P.; Echave, J.; Pereira, A.G.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Agriculture waste valorisation as a source of antioxidant phenolic compounds within a circular and sustainable bioeconomy. Food Funct. 2020. [Google Scholar] [CrossRef]
- Kheirkhah, H.; Baroutian, S.; Young, S. Food and Bioproducts Processing Evaluation of bioactive compounds extracted from Hayward kiwifruit pomace by subcritical water extraction. Food Bioprod. Process. 2019, 115, 143–153. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Oszmian, J. Phytochemical compounds and biological effects of Actinidia fruits. J. Funct. Foods 2017, 30, 194–202. [Google Scholar] [CrossRef]
- Bandara, N.; Chalamaiah, M. Bioactives from agricultural processing by-products. Encycl. Food Chem. 2018, 472–480. [Google Scholar] [CrossRef]
- Skinner, S.J.M.; Hunter, D.; Cho, S.; Skinner, M. The Potential Health Benefits of the Subtropical Fruits Kiwifruit, Feijoa and Tamarillo. In Bioactives in Fruit: Health Benefits and Functional Foods; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2013; pp. 169–195. [Google Scholar] [CrossRef]
- Dias, M.; Caleja, C.; Pereira, C.; Calhelha, R.C.; Kostic, M.; Sokovic, M.; Tavares, D.; José, I.; Barros, L.; Ferreira, I.C.F.R. Chemical composition and bioactive properties of byproducts from two di ff erent kiwi varieties. Food Res. Int. 2020, 127, 108753. [Google Scholar] [CrossRef]
- Yang, H.; Lee, Y.; Han, K.; Singh, H.; Yoon, M.; Park, J.; Cho, C.; Cho, S. Green and gold kiwifruit peel ethanol extracts potentiate pentobarbital-induced sleep in mice via a GABAergic mechanism. Food Chem. 2013, 136, 160–163. [Google Scholar] [CrossRef]
- He, X.; Fang, J.; Chen, X.; Zhao, Z.; Li, Y.; Meng, Y.; Huang, L. Actinidia chinensis Planch: A Review of Chemistry and Pharmacology. Front Pharmacol. 2019, 10, 1–18. [Google Scholar] [CrossRef]
- Picchi, G.; Lombardini, C.; Pari, L.; Spinelli, R. Physical and chemical characteristics of renewable fuel obtained from pruning residues. J. Clean. Prod. 2018, 171, 457–463. [Google Scholar] [CrossRef]
- Almeida, D.; Pinto, D.; Santos, J.; Vinha, A.F.; Palmeira, J.; Ferreira, H.N.; Rodrigues, F.; Oliveira, M.B.P.P. Hardy kiwifruit leaves (Actinidia arguta): An extraordinary source of value-added compounds for food industry. Food Chem. 2018, 259, 113–121. [Google Scholar] [CrossRef]
- Manzone, M.; Gioelli, F.; Balsari, P. Kiwi clear-cut: First evaluation of recovered biomass for energy production. Energies 2017, 10, 1837. [Google Scholar] [CrossRef]
- Marangi, F.; Pinto, D.; De Francisco, L.; Alves, R.C.; Puga, H.; Sut, S.; Dall, S.; Rodrigues, F.; Oliveira, M.B.P.P. Hardy kiwi leaves extracted by multi-frequency multimode modulated technology: A sustainable and promising by-product for industry. Food Res. Int. 2018, 112, 184–191. [Google Scholar] [CrossRef]
- Salinero, C.; Sainz, M.J. EFA 40/05: Técnicas de cultivo de kiwi. Estac. Fitopatol. Areeiro 2005, 4, 4–5. [Google Scholar]
- Trinh, L.T.P.; Choi, Y.S.; Bae, H.J. Production of phenolic compounds and biosugars from flower resources via several extraction processes. Ind. Crops Prod. 2018, 125, 261–268. [Google Scholar] [CrossRef]
- Leontowicz, H.; Leontowicz, M.; Latocha, P.; Jesion, I.; Park, Y.S.; Katrich, E.; Barasch, D.; Nemirovski, A.; Gorinstein, S. Bioactivity and nutritional properties of hardy kiwi fruit Actinidia arguta in comparison with Actinidia deliciosa “Hayward” and Actinidia eriantha “Bidan”. Food Chem. 2016, 196, 281–291. [Google Scholar] [CrossRef] [PubMed]
- D’Evoli, L.; Moscatello, S.; Lucarini, M.; Aguzzi, A.; Gabrielli, P.; Proietti, S.; Battistelli, A.; Famiani, F.; Böhm, V.; Lombardi-Boccia, G. Nutritional traits and antioxidant capacity of kiwifruit (Actinidia deliciosa Planch., cv. Hayward) grown in Italy. J. Food Compos. Anal. 2015, 37, 25–29. [Google Scholar] [CrossRef]
- Kleszczyn, H.; Cyboran, S.; Oszmian, J. Chemico-Biological Interactions Modification of the properties of biological membrane and its protection against oxidation by Actinidia arguta leaf extract. Chem. Biol. Interact. 2014, 222, 50–59. [Google Scholar] [CrossRef]
- Deng, J.; Liu, Q.; Zhang, C.; Cao, W.; Fan, D.; Yang, H. Extraction optimization of polyphenols from waste kiwi fruit seeds (Actinidia chinensis Planch.) and evaluation of its antioxidant and anti-inflammatory properties. Molecules 2016, 21, 832. [Google Scholar] [CrossRef]
- Alim, A.; Li, T.; Nisar, T.; Ren, D.; Zhai, X.; Pang, Y.; Yang, X. Antioxidant, antimicrobial, and antiproliferative activity-based comparative study of peel and flesh polyphenols from actinidia chinensis. Food Nutr. Res. 2019, 63, 1–10. [Google Scholar] [CrossRef]
- Lee, J.; Sowndhararajan, K.; Kim, M.; Kim, J.; Kim, D.; Kim, S.; Kim, G.; Kim, S.; Jhoo, J. Antioxidant, inhibition of a -glucosidase and suppression of nitric oxide production in LPS-induced murine macrophages by different fractions of Actinidia arguta stem. Saudi J. Biol. Sci. 2014, 21, 532–538. [Google Scholar] [CrossRef]
- Ansell, J.; Butts, C.A.; Paturi, G.; Eady, S.L.; Wallace, A.J.; Hedderley, D.; Gearry, R.B. Kiwifruit-derived supplements increase stool frequency in healthy adults: A randomized, double-blind, placebo-controlled study. Nutr. Res. 2015, 35, 401–408. [Google Scholar] [CrossRef]
- Drzewiecki, J.; Latocha, P.; Leontowicz, H.; Leontowicz, M.; Park, Y.S.; Najman, K.; Weisz, M.; Ezra, A.; Gorinstein, S. Analytical Methods Applied to Characterization of Actinidia arguta, Actinidia deliciosa, and Actinidia eriantha Kiwi Fruit Cultivars. Food Anal. Methods 2016, 9, 1353–1366. [Google Scholar] [CrossRef][Green Version]
- Nishimura, M.; Okimasu, Y.; Miyake, N.; Tada, M.; Hida, R.; Negishi, T. Inhibitory effect of Actinidia arguta on mutagenesis, inflammation and two-stage mouse skin tumorigenesis. Genes Environ. 2016, 38, 25. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.C.; Skinner, M.A.; Ferguson, A.R. Kiwifruit and health. In Fruits, Vegetables, and Herbs: Bioactive Foods in Health Promotion; Elsevier: Amsterdam, The Netherlands, 2016; pp. 239–269. ISBN 9780128029893. [Google Scholar]
- Cassano, A.; Donato, L.; Conidi, C.; Drioli, E. Recovery of bioactive compounds in kiwifruit juice by ultrafiltration. Innov. Food Sci. Emerg. Technol. 2008, 9, 556–562. [Google Scholar] [CrossRef]
- Cunha, L.C.M.; Lúcia, M.; Monteiro, G.; Lorenzo, J.M.; Munekata, P.E.S.; Muchenje, V.; Allan, F.; De Carvalho, L.; Conte-junior, C.A. Natural antioxidants in processing and storage stability of sheep and goat meat products. Food Res. Int. 2018, 111, 379–390. [Google Scholar] [CrossRef]
- Martin, H.; Cordiner, S.B.; Mcghie, T.K. Function Kiwifruit actinidin digests salivary amylase but not gastric lipase. Food Funct. 2017, 8, 3339–3345. [Google Scholar] [CrossRef] [PubMed]
- Güzel, M.; Akpınar, Ö. Food and Bioproducts Processing Valorisation of fruit by-products: Production characterization of pectins from fruit peels. Food Bioprod. Process. 2019, 115, 126–133. [Google Scholar] [CrossRef]
- Chai, W.; Shi, Y.; Feng, H.; Xu, L.; Xiang, Z.; Gao, Y.; Chen, Q. Structure Characterization and Anti-tyrosinase Mechanism of Polymeric Proanthocyanidins Fractionated from Kiwifruit Pericarp. J. Agric. Food Chem. 2014, 62, 6382–6389. [Google Scholar] [CrossRef]
- Moreira, D.M.; Ferreira, V.; Resende, P.R.; Pinho, C. Determination of kinetic data through the fluidized bed combustion of chars made from vine and kiwi pruning wastes. Energy Rep. 2020, 6, 615–619. [Google Scholar] [CrossRef]
| Composition | Chemical Structure | Res- | Ref. | Composition | Chemical Structure | Res- | Ref. |
|---|---|---|---|---|---|---|---|
| Flavanols (derived from quercetin and kaempferol) | Kaempferol-3-O-galactoside | P | [13] | Caffeoliquinic acid | P | [25] | |
| L | [3,27] | Di-O-caffeoylquinic acid | P | [25] | |||
| Quercetin-3-O-rutinóside | P | [13] | Epigallocatechin gallate | P | [25] | ||
| Other flavanols | Epicatechin | Sk | [16] | Procatechuic acid | Se | [28] | |
| L | [3,27] | P-coumaric acid | Se | [28] | |||
| Catechin | Sk | [29] | L | [3,27] | |||
| L | [3,27] | Ferulic acid | Se | [28] | |||
| B | [3,1,30] | Tocopherols | α-tocopherol | Sk | [16] | ||
| Quercetin | Sk | [16] | β-tocopherol | Sk | [16] | ||
| Flavan-3-ols | Polymeric procyanidins | P | [13] | γ-tocopherol | Sk | [16] | |
| Procyanidin dimer type B | L | [3,27] | Se | [28] | |||
| Phenolic acids | Caffeic acid | P | [13] | ƍ-tocotrienol | Se | [18] | |
| Se | [28] | Organic acids | Malic acid | Sk | [16] | ||
| Chlorogenic acid | P | [13] | Citric acid | Sk | [16] | ||
| L | [3,27] | Ascorbic acid | Sk | [16] | |||
| Quinic acid | P, Sk | [13,16] | Fatty acids | Linoleic acid | Se | [28] | |
| Anthocyanins | Cyanidin-3-O-sambubioside | P | [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamorro, F.; Carpena, M.; Nuñez-Estevez, B.; Prieto, M.A.; Simal-Gandara, J. Valorization of Kiwi by-Products for the Recovery of Bioactive Compounds: Circular Economy Model. Proceedings 2021, 70, 9. https://doi.org/10.3390/foods_2020-07647
Chamorro F, Carpena M, Nuñez-Estevez B, Prieto MA, Simal-Gandara J. Valorization of Kiwi by-Products for the Recovery of Bioactive Compounds: Circular Economy Model. Proceedings. 2021; 70(1):9. https://doi.org/10.3390/foods_2020-07647
Chicago/Turabian StyleChamorro, Franklin, María Carpena, Bernabé Nuñez-Estevez, Miguel A. Prieto, and Jesus Simal-Gandara. 2021. "Valorization of Kiwi by-Products for the Recovery of Bioactive Compounds: Circular Economy Model" Proceedings 70, no. 1: 9. https://doi.org/10.3390/foods_2020-07647
APA StyleChamorro, F., Carpena, M., Nuñez-Estevez, B., Prieto, M. A., & Simal-Gandara, J. (2021). Valorization of Kiwi by-Products for the Recovery of Bioactive Compounds: Circular Economy Model. Proceedings, 70(1), 9. https://doi.org/10.3390/foods_2020-07647









