Comparative Study on the Inhibition of Acetylcholinesterase Activity by Hyptis marrubioides, Hyptis pectinata, and Hyptis suaveolens Methanolic Extracts †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Determination of AchE Inhibitory Activity
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Sayre, L.M.; Perry, G.; Smith, M.A. Oxidative Stress and Neurotoxicity. Chem. Res. Toxicol. 2008, 21, 172–188. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Varma, R. Antioxidant activity and Phytochemical analysis of Hyptis suaveolens (L.) Poit. J. Adv. Pharm. Educ. Res. 2013, 3, 541–549. [Google Scholar] [CrossRef]
- Mozhiyarasi, P.; Anuradha, R. A Study on Antioxidant Activity of Hyptis suaveolens (L) poit. Int. J. Curr. Microbiol. App. Sci. 2018, 7, 376–382. [Google Scholar] [CrossRef]
- Ghaffari, H.; Ghassam, B.J.; Chandra Nayaka, S. Antioxidant and neuroprotective activities of Hyptis suaveolens (L.) Poit. Against oxidative stress-induced neurotoxicity. Cell. Mol. Neurobiol. 2013, 34, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Paixão, M.S.; Melo, M.S.; Oliveira, M.G.; Santana, M.T.; Lima, A.C.; Damascena, N.P.; Dias, A.S.; Araujo, B.S.; Estevam, C.S.; Botelho, M.A.; et al. Hyptis pectinata: Redox Protection and Orofacial Antinociception. Phytother. Res. 2013, 27, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
- Lima, K.; Silva, O.; Figueira, M.E.; Pires, C.; Cruz, D.; Gomes, S.; Maurício, E.M.; Duarte, M.P. Influence of the in vitro gastrointestinal digestion on the antioxidant activity T of Artemisia gorgonum Webb and Hyptis pectinata (L.) Poit. infusions from Cape Verde. Food Res. Int. 2019, 115, 150–159. [Google Scholar] [CrossRef]
- Vilasboas-Campos, D.; Costa, M.D.; Teixeira-Castro, A.; Rios, R.; Bessa, C.; Dias, A.C.P.; Maciel, P. Neurotherapeutic effect of Hyptis spp. leaf extracts in Caenorhabditis elegans models of tauopathy and polyglutamine disease: Role of the glutathione redox cycle. Free Radic. Biol. Med. 2020. [Google Scholar] [CrossRef]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of Alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef]
- Houghton, P.J.; Ren, Y.; Howes, M.-J. Acetylcholinesterase inhibitors from plants and fungi. Nat. Prod. Rep. 2006, 23, 181–199. [Google Scholar] [CrossRef]
- Dos Santos, T.C.; Gomes, T.M.; Pinto, B.; Camara, A.L.; Paes, A. Naturally Occurring Acetylcholinesterase Inhibitors and Their Potential Use for Alzheimer’s Disease Therapy. Front. Pharmacol. 2018, 9, 1192. [Google Scholar] [CrossRef]
- Gülçin, I.; Scozzafava, A.; Supuran, S.T.; Koksal, Z.; Turkan, F.; Çetinkaya, S.; Bingöl, Z.; Huyut, Z.; Alwasel, S.H. Rosmarinic acid inhibits some metabolic enzymes including glutathione S-transferase, lactoperoxidase, acetylcholinesterase, butyrylcholinesterase and carbonic anhydrase isoenzymes. J. Enzyme Inhib. Med. Chem. 2016, 31, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Mathew, M.; Subramanian, S. In Vitro Screening for Anti-Cholinesterase and Antioxidant Activity of Methanolic Extracts of Ayurvedic Medicinal Plants Used for Cognitive Disorders. PLoS ONE 2014, 9, e86804. [Google Scholar] [CrossRef] [PubMed]
- Chaowuttiku, C.; Palanuvej, C.; Ruangrungsi, N. Quantification of chlorogenic acid, rosmarinic acid, and caffeic acid contents in selected Thai medicinal plants using RP-HPLC-DAD. Braz. J. Pharm. Sci. 2020, 56, e17547. [Google Scholar] [CrossRef]
- Vladimir-Knežević, S.; Blažeković, B.; Kindl, M.; Vladić, J.; Lower-Nedza, A.D.; Brantner, A.H. Acetylcholinesterase inhibitory, antioxidant and phytochemical properties of selected medicinal plants of the Lamiaceae family. Molecules 2014, 19, 767–782. [Google Scholar] [CrossRef]
- Szwajgier, D. Anticholinesterase activity of selected phenolic acids and flavonoids—Interaction testing in model solutions. Ann. Agric. Environ. Med. 2015, 22, 690–694. [Google Scholar] [CrossRef]
- Khan, H.; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Uriarte-Pueyo, I.; Calvo, M. Flavonoids as acetylcholinesterase inhibitors. Curr. Med. Chem. 2011, 18, 5289–5302. [Google Scholar] [CrossRef]
- Bakoyiannis, I.; Daskalopoulou, A.; Pergialiotis, V.; Perrea, D. Phytochemicals and cognitive health: Are flavonoids doing the trick? Biomed. Pharmacother. 2019, 109, 1488–1497. [Google Scholar] [CrossRef]
- Anggreani, E.; Lee, C.Y. Neuroprotective Effect of Chlorogenic Acids against Alzheimer’s Diseases. IJFS 2016. [Google Scholar] [CrossRef]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E.; et al. The Therapeutic Potential of Apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef]
- Magalhães, V.; Rios, R.; Dias, A. Cytoprotective effect of Hyptis spp. extracts against oxidative insults. Free Radic. Biol. Med. 2018, 120, S145. [Google Scholar] [CrossRef]
- Massoud, F.; Gauthier, S. Update on the pharmacological treatment of Alzheimer’s disease. Curr. Neuropharmacol. 2010, 8, 69–80. [Google Scholar] [CrossRef] [PubMed]
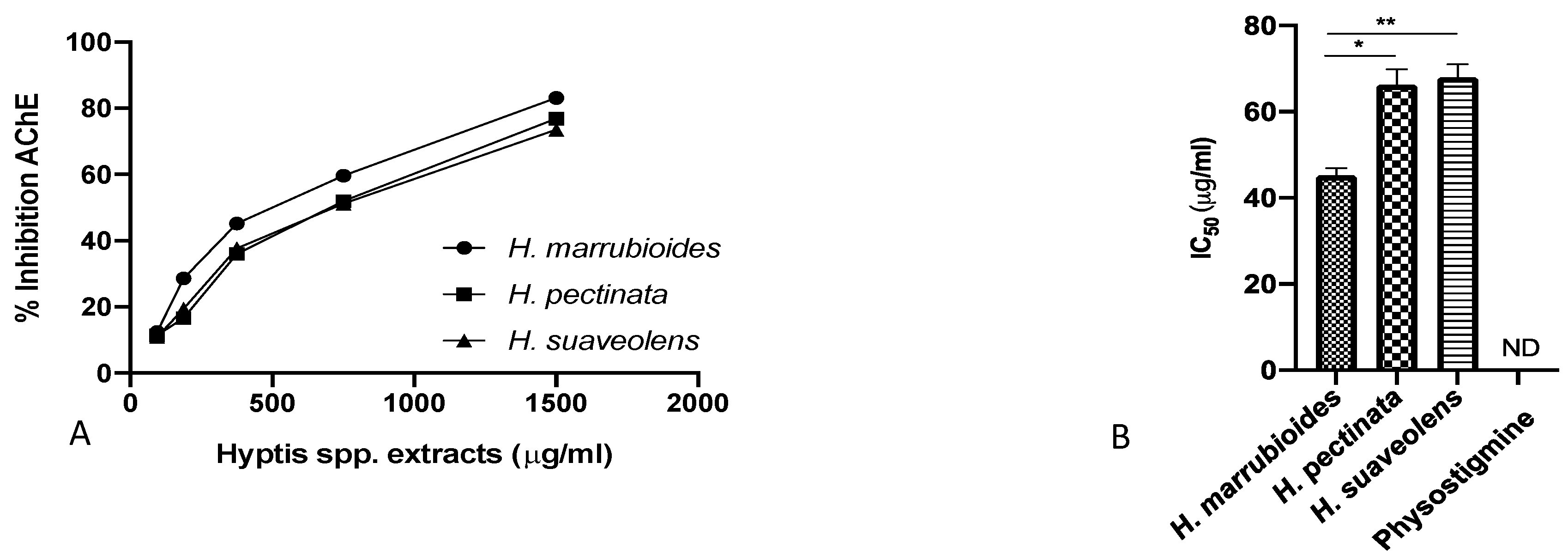
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magalhães, V.; Rios, R.M.; Silva, F.G.C.P.; Dias, A.C.P. Comparative Study on the Inhibition of Acetylcholinesterase Activity by Hyptis marrubioides, Hyptis pectinata, and Hyptis suaveolens Methanolic Extracts. Proceedings 2021, 70, 81. https://doi.org/10.3390/foods_2020-07628
Magalhães V, Rios RM, Silva FGCP, Dias ACP. Comparative Study on the Inhibition of Acetylcholinesterase Activity by Hyptis marrubioides, Hyptis pectinata, and Hyptis suaveolens Methanolic Extracts. Proceedings. 2021; 70(1):81. https://doi.org/10.3390/foods_2020-07628
Chicago/Turabian StyleMagalhães, Vanessa, Rejaine M. Rios, Fabiano G. C.P. Silva, and Alberto C. P. Dias. 2021. "Comparative Study on the Inhibition of Acetylcholinesterase Activity by Hyptis marrubioides, Hyptis pectinata, and Hyptis suaveolens Methanolic Extracts" Proceedings 70, no. 1: 81. https://doi.org/10.3390/foods_2020-07628
APA StyleMagalhães, V., Rios, R. M., Silva, F. G. C. P., & Dias, A. C. P. (2021). Comparative Study on the Inhibition of Acetylcholinesterase Activity by Hyptis marrubioides, Hyptis pectinata, and Hyptis suaveolens Methanolic Extracts. Proceedings, 70(1), 81. https://doi.org/10.3390/foods_2020-07628





