Decontamination of Pig Carcasses with Organic Acids †
Abstract
1. Introduction
2. Material and Methods
2.1. Inoculum Preparation
2.2. Sampling
2.3. Inoculation and Decontamination Procedure
2.4. Enumeration of Salmonella spp.
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
References
- van Ba, H.; Seo, H.W.; Pil-Nam, S.; Kim, Y.S.; Park, B.Y.; Moon, S.S.; Kang, S.J.; Choi, Y.M.; Kim, J.H. The effects of pre-and post-slaughter spray application with organic acids on microbial population reductions on beef carcasses. Meat Sci. 2018, 137, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Food, E.; Authority, S. The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17. [Google Scholar] [CrossRef]
- Borch, E.; Arinder, P. Bacteriological safety issues in red meat and ready-to-eat meat products, as well as control measures. Meat Sci. 2002, 62, 381–390. [Google Scholar] [CrossRef]
- Mani-López, E.; García, H.S.; López-Malo, A. Organic acids as antimicrobials to control Salmonella in meat and poultry products. Food Res. Int. 2012, 45, 713–721. [Google Scholar] [CrossRef]
- Ellatif, Z.A.; Saad, S.; Hassanin, F.; Salem, A.; Saleh, E. Efficiency of some organic acids as decontaminants in sheep carcasses. Benha Vet. Med. J. 2020, 38, 116–119. [Google Scholar] [CrossRef]
- Bonardi, S.; Bruini, I.; Alpigiani, I.; Vismarra, A.; Barilli, E.; Brindani, F.; Morganti, M.; Bellotti, P.; Bolzoni, L.; Pongolini, S. Influence of pigskin on Salmonella contamination of pig carcasses and cutting lines in an Italian slaughterhouse. Ital. J. Food Saf. 2016, 5, 5654. [Google Scholar] [CrossRef] [PubMed]
- Hugas, M.; Tsigarida, E. MEAT Pros and cons of carcass decontamination: The role of the European Food Safety Authority. Meat Sci. 2008, 78, 43–52. [Google Scholar] [CrossRef] [PubMed]
- van Immerseel, F.; Russell, J.B.; Flythe, M.D.; Gantois, I.; Timbermont, L.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R. The use of organic acids to combat Salmonella in poultry: A mechanistic explanation of the efficacy. Avian Pathol. 2006, 35, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Dan, S.D.; Mihaiu, M.; Reget, O.; Oltean, D.; Tăbăran, A. Pathogens Contamination Level Reduction on Beef Using Organic Acids Decontamination Methods. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca. Vet. Med. 2017, 74, 212. [Google Scholar] [CrossRef][Green Version]
- Yeh, Y.; de Moura, F.H.; van den Broek, K.; de Mello, A.S. Effect of ultraviolet light, organic acids, and bacteriophage on Salmonella populations in ground beef. Meat Sci. 2018, 139, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Madushanka, D.N.N.; Jayaweera, T.S.P.; Jayasinghe, J.M.C.S.; Yasawathie, D.G.; Ruwandeepika, H.A.D. Decontaminating Effect of Organic Acids and Natural Compounds on Broiler Chicken Meat Contaminated with Salmonella typhimurium. Asian Food Sci. J. 2018, 3, 1–9. [Google Scholar] [CrossRef]

| Suspension A | Control | Lactic Acid 2% | Lactic Acid 5% | Citric Acid 2% | Citric Acid 5% | Effect |
|---|---|---|---|---|---|---|
| 30 min | 4.06 ± 0.05 a | 4.23 ± 0.25 | 4.12 ± 0.13 | 4.28 ± 0.61 | 4.20 ± 0.42 | n.s. |
| 6 h | 4.14 ± 0.06 a | 3.98 ± 0.33 | 4.21 ± 0.37 | 4.25 ± 0.70 | 4.22 ± 0.38 | n.s. |
| 12 h | 4.84 ± 0.62 a,b | 4.44 ± 0.22 | 4.33 ± 0.23 | 4.48 ± 0.21 | 4.56 ± 0.35 | n.s. |
| 24 h | 5.39 ± 0.20 b,A | 4.27 ± 0.28 B | 4.13 ± 0.17 B | 4.36 ± 0.20 B | 4.24 ± 0.17 B | *** |
| 48 h | 5.42 ± 0.03 b | 4.88 ± 0.52 | 4.63 ± 0.78 | 5.13 ± 0.23 | 4.86 ± 0.64 | n.s. |
| Effect | *** | n.s. | n.s. | n.s. | n.s. |
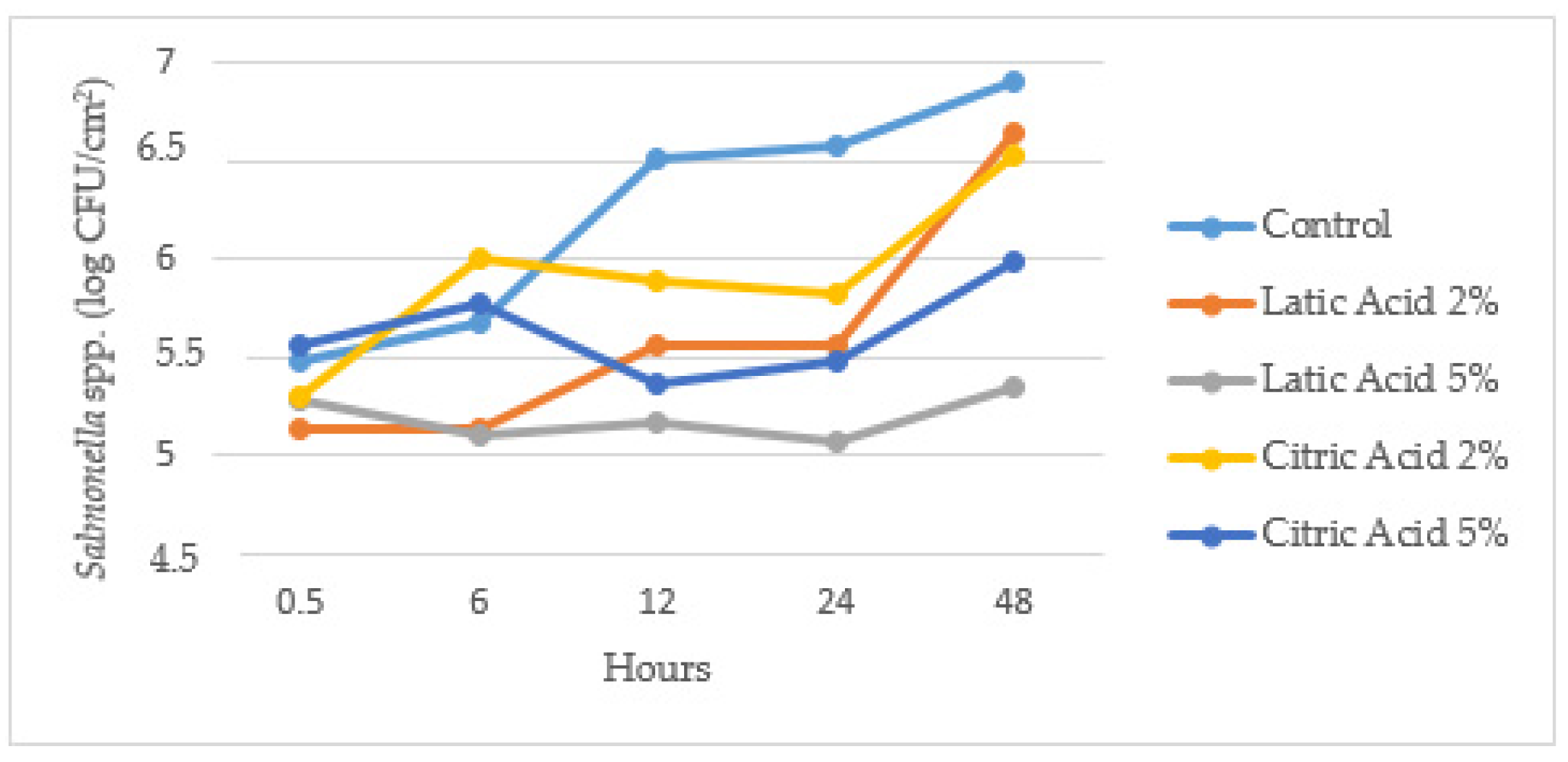
| Suspension B | Control | Lactic Acid 2% | Lactic Acid 5% | Citric Acid 2% | Citric Acid 5% | Effect |
|---|---|---|---|---|---|---|
| 30 min | 5.48 ± 0.07 ª | 5.14 ± 0.24 ª | 5.12 ± 0.16 | 5.29 ± 0.20 ª | 5.56 ± 0.94 | n.s. |
| 6 h | 5.67 ± 0.14 ª | 5.14 ± 0.75 ª | 5.10 ± 0.62 | 6.01 ± 0.17 ªb | 5.78 ± 0.11 | n.s. |
| 12 h | 6.51 ± 0.08 b,A | 5.56 ± 0.31 a,b,A,B | 5.17 ± 0.42 B | 5.90 ± 0.28 a,b,A,B | 5.37 ± 0.53 B | ** |
| 24 h | 6.57 ± 0.20 b,c,A | 5.56 ± 0.25 a,b,B,C | 5.07 ± 0.16 C | 5.83 ± 0.22 a,b,B | 5.47 ± 0.07 B,C | *** |
| 48 h | 6.91 ± 0.10 c,A | 6.64 ± 0.27 b,A,B,C | 5.35 ± 0.32 | 6.52 ± 0.53 b,A,B,C | 5.98 ± 0.19 B | *** |
| Effect | *** | ** | n.s. | ** | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciríaco, M.; Moura-Alves, M.; Silva, R.; Pinto, I.; Saraiva, C.M.; Esteves, A. Decontamination of Pig Carcasses with Organic Acids. Proceedings 2021, 70, 63. https://doi.org/10.3390/foods_2020-07649
Ciríaco M, Moura-Alves M, Silva R, Pinto I, Saraiva CM, Esteves A. Decontamination of Pig Carcasses with Organic Acids. Proceedings. 2021; 70(1):63. https://doi.org/10.3390/foods_2020-07649
Chicago/Turabian StyleCiríaco, Maria, Márcio Moura-Alves, Rui Silva, Isabel Pinto, Cristina Maria Saraiva, and Alexandra Esteves. 2021. "Decontamination of Pig Carcasses with Organic Acids" Proceedings 70, no. 1: 63. https://doi.org/10.3390/foods_2020-07649
APA StyleCiríaco, M., Moura-Alves, M., Silva, R., Pinto, I., Saraiva, C. M., & Esteves, A. (2021). Decontamination of Pig Carcasses with Organic Acids. Proceedings, 70(1), 63. https://doi.org/10.3390/foods_2020-07649






