Extraction, Chemical Characterization, and Antioxidant Activity of Bioactive Plant Extracts †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extraction Procedures
2.2. Total Phenolic and Flavonoid Content Determinations
2.3. Antioxidant Activity Determination
2.4. Identification and Quantification of Individual Phenolic Compounds
2.5. Statistical Analysis
3. Results and Discussion
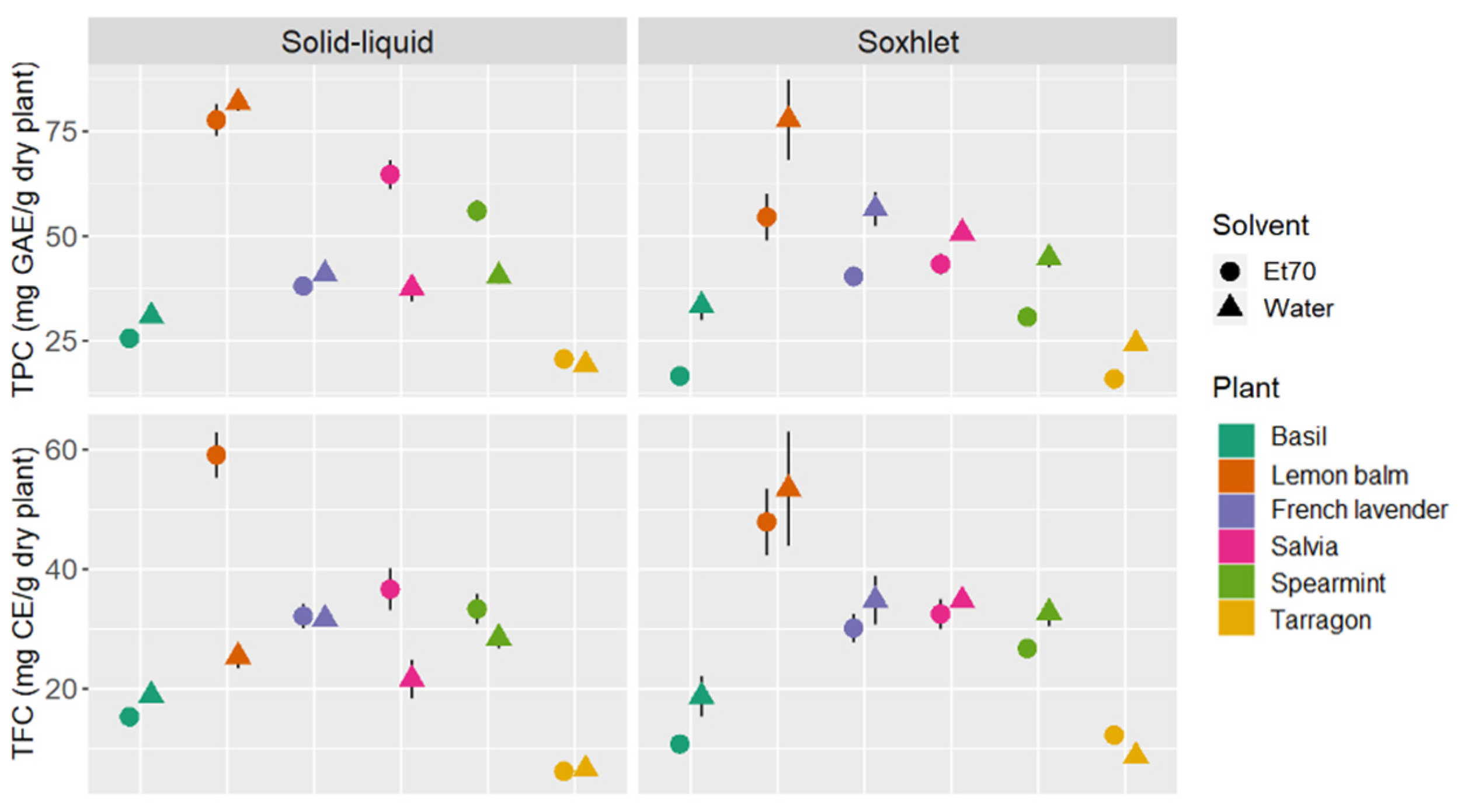
3.1. Total Phenolic Content and Total Flavonoid Content
3.2. Antioxidant Activity
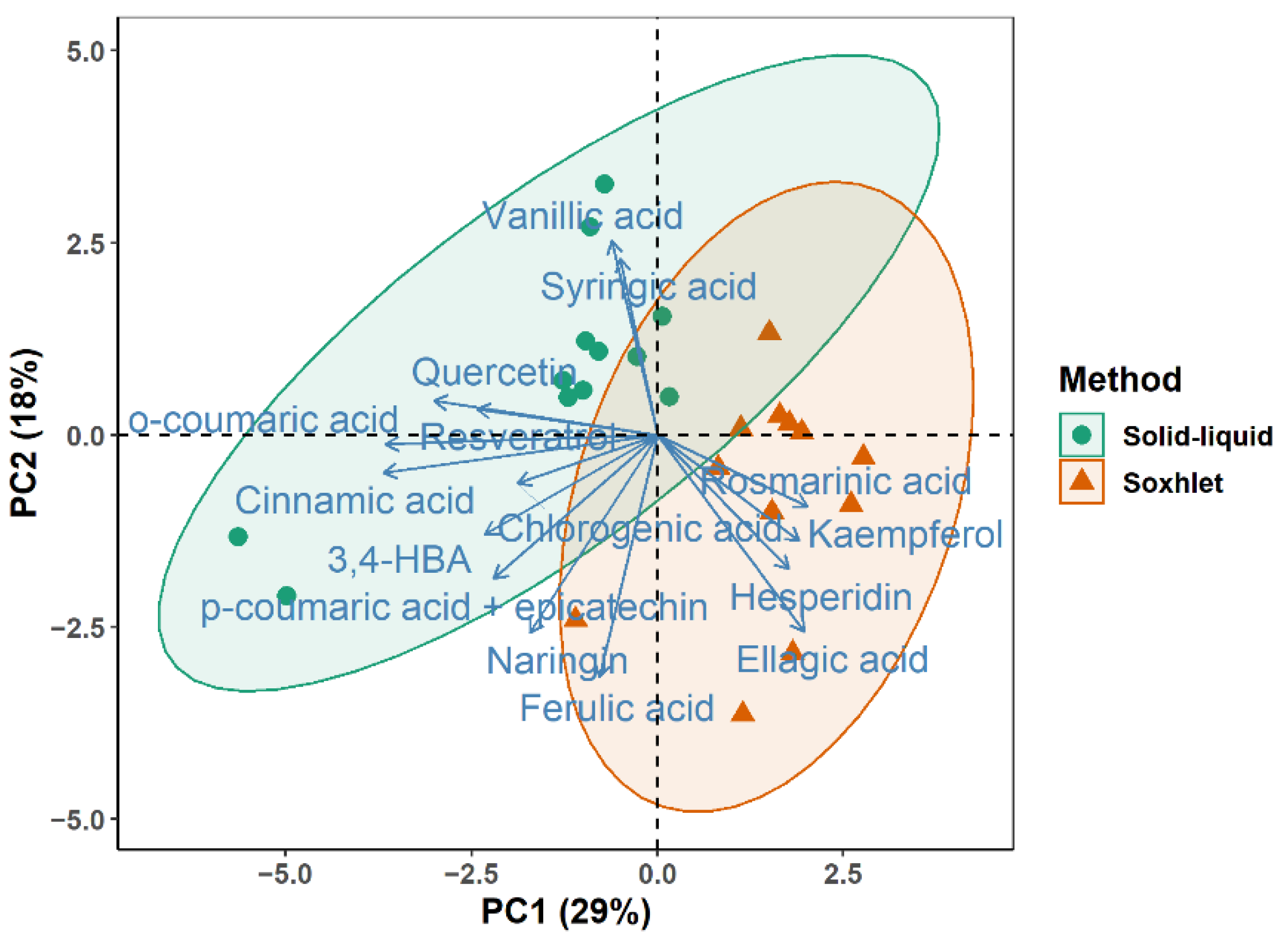
3.3. Identification and Quantification of Individual Phenolic Compounds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Antolak, H.; Kregiel, D. Food Preservatives from Plants. In Food Additives; Karunaratne, D.N., Pamunuwa, G., Eds.; IntechOpen: London, UK, 2017; pp. 45–85. [Google Scholar]
- Chibane, L.B.; Degraeve, P.; Ferhout, H.; Bouajila, J.; Oulahal, N. Plant antimicrobial polyphenols as potential natural food preservatives. J. Sci. Food Agric. 2019, 99, 1457–1474. [Google Scholar] [CrossRef] [PubMed]
- Regnier, T.; Combrinck, S.; Du Plooy, W. Essential Oils and Other Plant Extracts as Food Preservatives. In Progress in Food Preservation; Bhat, R., Karim Alias, A., Paliyath, G., Eds.; Wiley-Blackwell: Oxford, UK, 2012; pp. 539–579. [Google Scholar]
- Sharifi-Rad, M.; Ozcelik, B.; Altın, G.; Daşkaya-Dikmen, C.; Martorell, M.; Ramírez-Alarcón, K.; Alarcón-Zapata, P.; Morais-Braga, M.F.B.; Carneiro, J.N.P.; Leal, A.L.A.B.; et al. Salvia spp. plants-from farm to food applications and phytopharmacotherapy. Trends Food Sci. Technol. 2018, 80, 242–263. [Google Scholar] [CrossRef]
- Carocho, M.; Barreira, J.C.M.; Bento, A.; Fernández-Ruiz, V.; Morales, P.; Ferreira, I.C.F.R. Chestnut and lemon balm based ingredients as natural preserving agents of the nutritional profile in matured “Serra da Estrela” cheese. Food Chem. 2019, 204, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, B.A.; Shahidi, F.; Yazdi, F.T.; Mortazavi, S.A.; Mohebbi, M. Antioxidant activity and antimicrobial effect of tarragon (Artemisia dracunculus) extract and chemical composition of its essential oil. J. Food Meas. Charact. 2017, 11, 847–863. [Google Scholar] [CrossRef]
- Mussatto, S.I. Generating Biomedical Polyphenolic Compounds from Spent Coffee or Silverskin. In Coffee in Health and Disease Prevention; Preedy, V.R., Ed.; Elsevier Inc.: London, UK, 2015; pp. 93–106. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Meth. Enzymol. 1999, 299, 152–178. [Google Scholar]
- Barros, L.; Oliveira, S.; Carvalho, A.M.; Ferreira, I.C.F.R. In vitro antioxidant properties and characterization in nutrients and phytochemicals of six medicinal plants from the Portuguese folk medicine. Ind. Crops Prod. 2010, 32, 572–579. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Cerqueira, M.A.; Teixeira, J.A.; Mussatto, S.I. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohydr. Polym. 2015, 127, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.I. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef]
- Seabra, I.J.; Dias, A.M.A.; Braga, M.E.M.; de Sousa, H.C. High pressure solvent extraction of maritime pine bark: Study of fractionation, solvent flow rate and solvent composition. J. Supercrit. Fluids 2012, 62, 135–148. [Google Scholar] [CrossRef]
- Bai, X.-L.; Yue, T.-L.; Yuan, Y.-H.; Zhang, H.-W. Optimization of microwave-assisted extraction of polyphenols from apple pomace using response surface methodology and HPLC analysis. J. Sep. Sci. 2010, 33, 3751–3758. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Genisheva, Z.; Pereira, R.N.; Teixeira, J.A.; Rocha, C.M.R. Moderate electric fields as a potential tool for sustainable recovery of phenolic compounds from Pinus pinaster bark. ACS Sustain. Chem. Eng. 2019, 7, 8816–8826. [Google Scholar] [CrossRef]
| Extraction Method/Solvent | |||||
|---|---|---|---|---|---|
| Plant | Solid-Liquid | Soxhlet | |||
| Water | Et70 | Water | Et70 | ||
| FRAP (μmol Fe2+/g dry plant) | Basil | 400.5 ± 9.76 | 342.5 ± 11.7 | 508.6 ± 19.7 | 256.5 ± 1.52 |
| Lemon balm | 692.8 ± 97.7 | 1166 ± 34.3 | 1182 ± 126 | 1094 ± 22.9 | |
| French lavender | 478.1 ± 112 | 682.4 ± 39.8 | 818.0 ± 65.5 | 511.2 ± 80.6 | |
| Salvia | 561.9 ± 14.7 | 846.4 ± 44.7 | 791.3 ± 35.0 | 857.2 ± 66.7 | |
| Spearmint | 621.1 ± 65.6 | 808.1 ± 57.2 | 776.9 ± 8.57 | 688.6 ± 36.3 | |
| Tarragon | 143.2 ± 2.72 | 202.5 ± 6.45 | 232.2 ± 3.71 | 204.4 ± 19.1 | |
| DPPH (μmol TE/g dry plant) | Basil | 157.4 ± 10.6 | 152.8 ± 10.0 | 193.0 ± 1.20 | 85.38 ± 0.97 |
| Lemon balm | 302.9 ± 29.0 | 365.3 ± 0.98 | 362.8 ± 19.5 | 359.8 ± 2.36 | |
| French lavender | 183.2 ± 54.8 | 282.8 ± 11.0 | 292.6 ± 37.5 | 212.8 ± 41.6 | |
| Salvia | 210.5 ± 9.78 | 309.9 ± 7.84 | 265.7 ± 0.50 | 280.8 ± 23.8 | |
| Spearmint | 219.6 ± 28.0 | 312.0 ± 21.3 | 268.1 ± 0.50 | 231.5 ± 12.8 | |
| Tarragon | 57.84 ± 1.54 | 42.95 ± 14.1 | 94.90 ± 1.50 | 62.95 ± 1.07 | |
| ABTS (μmol TE/g dry plant) | Basil | 197.0 ± 7.90 | 216.5 ± 6.84 | 227.3 ± 3.48 | 122.6 ± 0.98 |
| Lemon balm | 410.0 ± 75.3 | 596.2 ± 15.4 | 532.8 ± 31.1 | 492.1 ± 8.12 | |
| French lavender | 261.9 ± 63.3 | 381.4 ± 15.4 | 389.1 ± 51.9 | 276.9 ± 52.9 | |
| Salvia | 264.8 ± 3.16 | 436.8 ± 11.4 | 382.7 ± 4.72 | 356.9 ± 27.6 | |
| Spearmint | 323.9 ± 30.9 | 434.5 ± 31.0 | 379.2 ± 6.21 | 289.2 ± 10.1 | |
| Tarragon | 86.96 ± 2.24 | 114.2 ± 3.71 | 130.4 ± 4.47 | 102.1 ± 19.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, B.N.; Cadavez, V.; Ferreira-Santos, P.; Teixeira, J.A.; Gonzales-Barron, U. Extraction, Chemical Characterization, and Antioxidant Activity of Bioactive Plant Extracts. Proceedings 2021, 70, 62. https://doi.org/10.3390/foods_2020-07739
Silva BN, Cadavez V, Ferreira-Santos P, Teixeira JA, Gonzales-Barron U. Extraction, Chemical Characterization, and Antioxidant Activity of Bioactive Plant Extracts. Proceedings. 2021; 70(1):62. https://doi.org/10.3390/foods_2020-07739
Chicago/Turabian StyleSilva, Beatriz Nunes, Vasco Cadavez, Pedro Ferreira-Santos, José António Teixeira, and Ursula Gonzales-Barron. 2021. "Extraction, Chemical Characterization, and Antioxidant Activity of Bioactive Plant Extracts" Proceedings 70, no. 1: 62. https://doi.org/10.3390/foods_2020-07739
APA StyleSilva, B. N., Cadavez, V., Ferreira-Santos, P., Teixeira, J. A., & Gonzales-Barron, U. (2021). Extraction, Chemical Characterization, and Antioxidant Activity of Bioactive Plant Extracts. Proceedings, 70(1), 62. https://doi.org/10.3390/foods_2020-07739







