1. Introduction
Development of biodegradable polymeric materials from renewable sources has gained a lot of attention with the growing environment pollution and energy shortage caused by the petroleum consumption [
1]. Due to the increasing awareness of sustainability, traditional plastic materials have become less attractive in comparison to more eco-friendly alternatives. Aliphatic polyesters seem to be a good candidate to aim this goal as they can be produced using monomers fully driven from biomass feedstock [
2]. They are suitable for conventional materials replacement due to the wide range of advantageous features, good mechanical properties and variable transition temperatures, thanks to which they can be proceeded into different forms. Among this group, segmented block copolyesters also need to be taken under consideration as they consist of different types of building blocks possessing various properties and transition temperatures, thus being capable of forming hard and soft sequences. Hard segments impart the dimensional, thermal and mechanical stability of the polymer, and the soft segments are responsible for the elasticity of the material. Proper selection of these building sequences makes it possible to obtain biobased copolyesters with desirable properties and a broad spectrum of potential applications such as: fibers, packaging, scaffolds and drug delivery systems [
3,
4,
5].
Lipases are a group of enzymes which are extensively explored in biocatalytic synthesis of polymeric materials, and
Candida antarctica lipase B (CAL-B) is an important member of this family as it possesses many beneficial features including: high regio-, chemio-, stereo- and enantioselectivity as well as catalytic activity, which enables one to obtain highly structure-regulated materials [
6,
7]. Additionally, CAL-B displays wide substrate specificity, and it works effectively under mild conditions with different monomers and solvents. According to the literature data, lipases can be successfully employed to produce polymeric materials such as: polyamides, polycarbonates, polyesters, etc. and be a good replacement for organometallic catalysts, which can have an undesirable impact on the environment [
8,
9,
10,
11]. All these facts combined show that biocatalysis is an effective platform for developing a “green” and sustainable polymer chemistry, and this approach is even more attractive since both substrates and catalysts are obtained from biomass [
4]. To extend the library of biobased copolyesters synthesized using enzymes as biocatalyst, we performed two-stage polycondensation of diethyl succinate, 1,4 – butanediol and dilinoleic acid diol (DLA-OH) in diphenyl ether. The obtained material was further characterized regarding the chemical structure, molecular weight, crystalline and thermal properties.
2. Experiments
2.1. Materials
The following materials were used in polymers preparation and characterization: dichloromethane (DCM: ≥99.5%), diphenyl ether (≥99%) acquired from Sigma Aldrich (Poznan, Poland), diethyl succinate (DS: ≥99%) acquired from Matrix Chemicals (Sevelen, Switzerland), 1,4-butanediol (BD: ≥99%) acquired from Alfa Aesar (Kandel, Germany), dimer linoleic diol (DLA-OH) PripolTM 2033 (dimer alcohol: ≥96.5%) obtained from Croda Coatings & Polymers (Gouda, The Netherlands), chloroform (CHCl3: ≥98.5%) acquired from Chempur (Piekary Slaskie, Poland), metanol (MeOH: ≥99.8%) acquired from Stanlab (Lublin, Polska). Candida antarctica type B lipase (CAL-B), immobilized on polyacrylate beads (300–500 µm > 95%, Fermase CALBTM 10,000, nominal activity of 10,000 PLU/g (propyl laurate Units per gram dry weight)), was acquired from Fermenta Biotech Ltd. Mumbai and from Enzyme Catalyzed Polymers LLC Akron (OH, USA).
2.2. Polymer Synthesis
Poly(butylene succinate-co-dilinoleic succinate) (PBS-DLS) copolyester with a 70:30 wt % ratio of hard-to-soft segments was synthesized via two-step melt polycondensation process. Soft segments (abbreviated as DLS) were fabricated using dilinoleic acid diol (DLA-OH) and diethyl succinate while hard segments (abbreviated as PBS) were manufactured using 1,4-butanediol and diethyl succinate. Concisely, CAL-B (10 wt % of all monomers), 1,4-butanediol, diethyl succinate, dilinoleic acid diol (DLA-OH) and diphenyl ether (200 wt % of all monomers) were weighted and transferred to a round-bottom flask, which was then placed into an oil-heated bath. In the first step, the reaction mixture was heated to 80 °C under argon flow and stirred for 1 h to obtain a homogeneous solution. After that time, the reaction mixture was heated to 95 °C, and the collection of by-product was monitored (3 h). In the next step, pressure of 600 Torr was applied to allow for conversion of monomers into oligomers (21 h, 95 °C). Further polymerization was carried out under reduced pressure of 2 Torr while keeping reaction temperature at 95 °C for 96 h. When the polymerization process was completed, the reaction mixture was dissolved in chloroform and enzyme was removed via filtration. Subsequently, the obtained product was precipitated with cold methanol and dried in vacuo (40 °C, 24 h).
2.3. Material Characterization
The chemical structure of the synthesized copolymer was assessed by nuclear magnetic resonance (1H NMR) and Fourier Transform infrared spectroscopy (ATR–FTIR). A TM Bruker DPX 400 spectrometer (400 MHz) was used to obtain spectra of 1H NMR (128 scans, 1 second relaxation delay). The samples were prepared in CDCl3 and tetramethylsilane (TMS) was used as internal reference. Attenuated Total Reflection–Fourier Transform Infrared (ATR–FTIR) spectra were recorded on Bruker APLHA spectrometer at spectral range from 400 to 4000 cm-1 with a resolution of 2 cm-1. Of note, 32 scans were performed for each sample.
The molecular weights (Mn and Mw) as well as dispersity index (Đ, Mw/Mn) were measured at 35 °C using a gel permeation chromatograph (GPC) equipped with a Viscotek VE 1122 pump, two 300 × 75 mm Styragel columns (PLgel 5 µm Mixed C, Polymer Laboratories) and detector (Shodex SE 61 Refractive Index Detector). Chloroform of HPCL grade was employed as the eluent, with a flow rate of 1 mL/min. The molecular weight calculations were conducted based on the universal calibration method using the universal calibration curve determined for narrow polydispersity polystyrene standards.
The thermal properties of the obtained PBS-DLS 70:30 copolyester were investigated by using TA-Instruments DSC Q2500 Discovery differential scanning calorimeter (DSC). The heating and cooling rates were 10 °C/min, and the measurements were performed over the temperature range from −90 to 200 °C. The glass transition temperature (Tg) was derived as a midpoint of the transition during a second heating cycle.
The cytotoxicity tests were performed via indirect contact according to ISO10993-5 using mouse fibroblast cells line L929. Next, 150 mg of PBS-DLS 70:30 copolymer were placed into a well of a 24-well culture plate. Then, 2 mL of complete growth media (Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% fetal bovine serum (FBS), 2 mM L-glutamine, 100 U/mL penicillin and 100 µg/mL streptomycin) were added to the well, and the plate was incubated in a CO2 incubator intended for cell culture at 37 °C for 24 hours. Simultaneously, a 96-well plate was prepared and 10,000 of L929 mouse fibroblast cells were plated at each well (5% CO2, 37 °C, 24 h). After 24 h of culture, the media was replaced with 100 µL of sample extract (6 technical repetitions were performed), and the plate was incubated again for 24 hours. After incubation, cell viability was determined by phase contrast microscopy and resazurin viability assay, where 20 mL of resazurin solution (0.15 mg/mL in PBS) were then added to the test well and the plates were transferred to the incubator (5% CO2, 37 °C) for another 4 hours. The fluorescence (Em: 540, Ex: 590) has been measured by using BioTek Synergy HTX multifunctional plate reader.
3. Results and Discussion
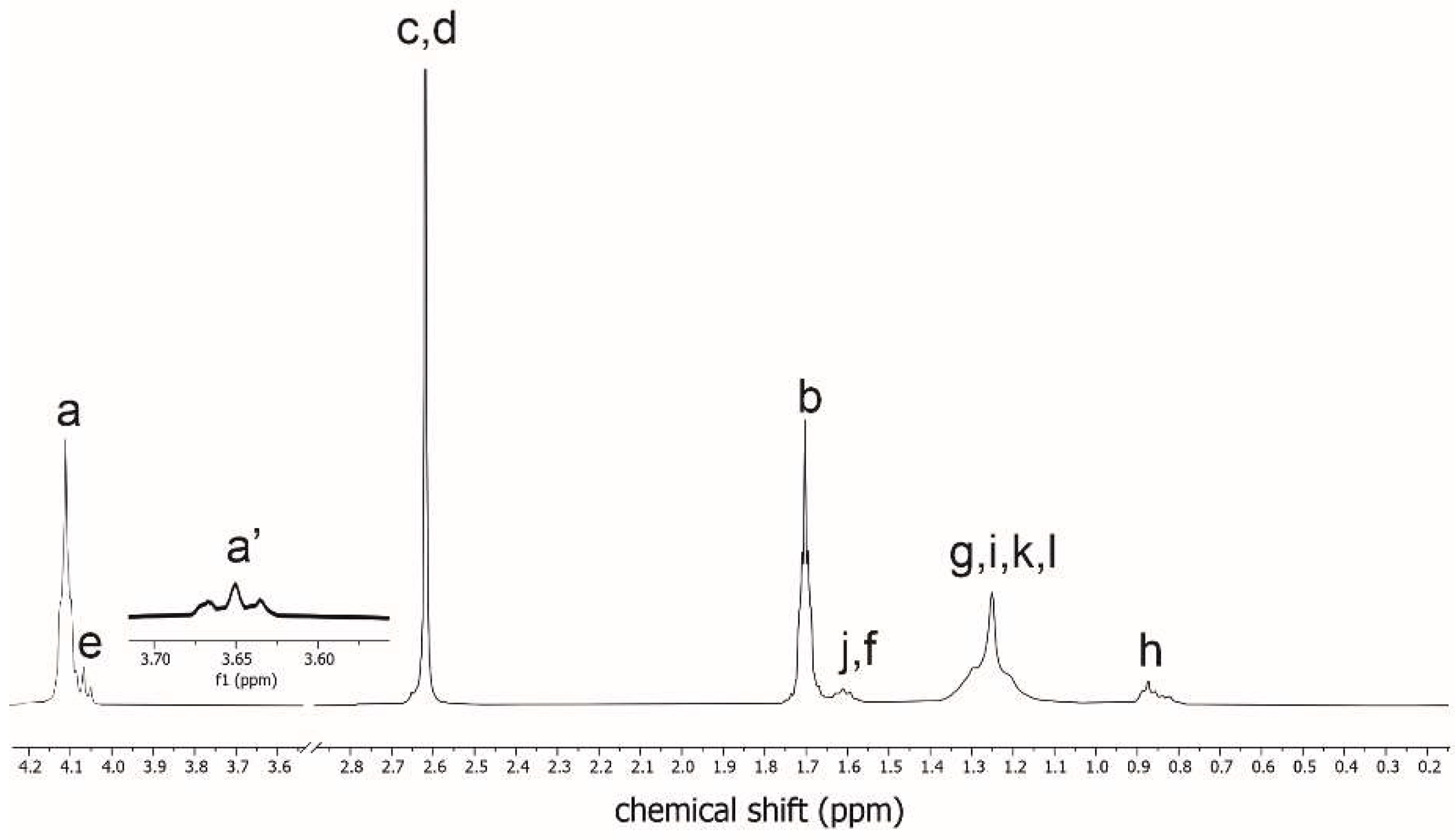
Nuclear magnetic resonance (NMR) has been used to verify the chemical structure of the obtained PBS-DLS copolyester. Structural analysis of 1H-NMR spectra and peaks assignments are presented in
Figure 1 and
Figure 2.
The characteristic signal assignments for PBS-DLS 70:30 copolyester were registered from 1H NMR (
Figure 2). Chemical shifts at δ1H = 4.12 ppm (a) indicated the creation of 1,4 butanediol-diethyl succinate ester bond, while chemical shifts at δ1H = 4.06–4.08 ppm (e) confirmed ester bond formation between dilinoleic acid diol and diethyl succinate. Protons from the methylene end groups of 1,4-butanediol in hard segments showed resonances at δ1H = 3.66–3.69 ppm (a’), while internal methylene protons on diethyl succinate and 1,4-butanediol are detected at δ1H = 2.63 ppm (c, d) and δ1H = 1.71 ppm (b), respectively. Signals located at δ1H = 0.85 ppm (h) are corresponding to the six protons on dilinoleic acid diol methylene end groups. Signals (f), (g), (i), (j), (k), (l) and (e) are confirming the presence of methylene groups in long aliphatic DLS chain. Based on 1H NMR measurements, the expected chemical structure of copolymer was confirmed and the sample composition was assessed following the method presented in [
12] and showed in
Table 1.
Table 1 summarizes the theoretical and final copolymer composition. As it can be observed, we were able to obtain good match between theoretical values and those estimated from 1H NMR spectra; however, a greater amount of soft DLS sequence has been implemented into copolymer, in comparison to the initial feed, and probably this may be caused by the evaporation of 1,4-butanediol during reaction when the vacuum is applied. DLA-OH possess longer aliphatic chain and greater molecular mass (540 g/mol). Therefore, we can assume that evaporation of this compound is less effective, and it remains in the reactor. Furthermore, the average molecular masses (Mn, Mw) and dispersity index (Đ) of PBS-DLS 70:30 copolyester were assessed by GPC measurements. Taking under consideration similar reported methods in which PBS-based copolymers were synthesized by biocatalysis [
2,
9], we obtain quite high Mn (25,200 g/mol) and Mw (205,600 g/mol) values; however, Đ value was thought to be more characteristic for addition–elimination reactions (equal or greater than 2), which is quite usual for step-growth polycondensation [
8]. Herein, dispersity index is much higher, which may indicate that CAL-B exhibit a tendency to create material with different macromolecules distribution and size.
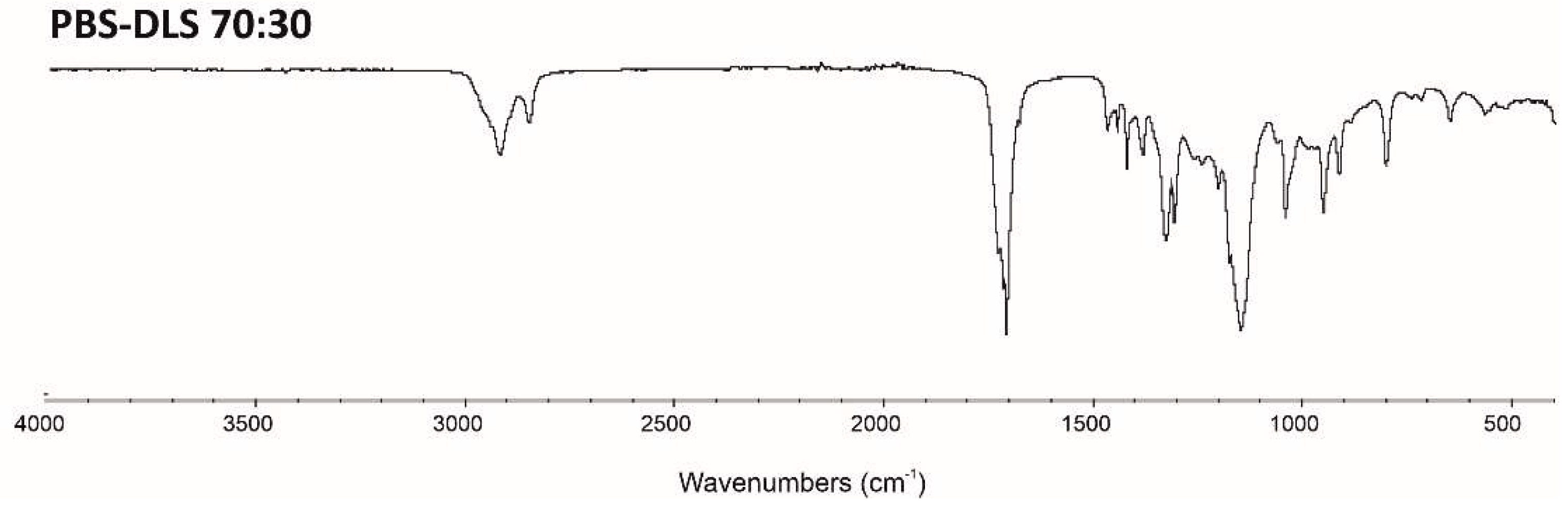
The ATR–FTIR spectra of PBS-DLS 70:30 copolymer is presented in
Figure 3. The characteristic absorption bands of the obtained material are assigned as follows. ATR–FTIR (cm-1): 2920 and 2855 (stretching vibrations of the -CH2), 1710 (C=O carbonyl vibrations), 1210 and 1150 (asymmetric and symmetric C-O-C stretching vibrations of ester groups, respectively), 1445–1470 (deformation vibrations of the -CH2- groups), 1390–1425 (wagging vibrations of the -CH2- groups), 1000–500 (in-plane and out-of-plane deformation vibrations of C-H and C-C groups).
The thermal properties of the PBS-DLS 70:30 copolymer were characterized with the use of DSC, and the results are presented in
Table 2. Distinct thermal transitions, such as T
g, T
m and T
c, were observed, which indicate material semicrystallinity. We found that glass transition temperature (T
g) of copolymer is noticeably lower in comparison to neat PBS sample, which proves that amorphous DLS soft segments were well incorporated into the material structure. Furthermore, T
c,
H
c and X
c values also decreased due to the presence of DLS sequences, which obviously are hindering the crystallization transition during cooling from the melt. This fact can be explained by the greater distances appearing between PBS rigid blocks, which are responsible for material crystallinity. Moreover, the addition of DLS soft segments contributed to the reduction in melting enthalpy (from 81 to 49.52 J/g) and T
m (from 110 to 98 °C), which also indicates that quality, perfection and degree of crystalline phase decreases due to the weaker intermolecular interactions [
13].
In order to assess the cytotoxicity of the synthesized PBS-DLS 70:30 copolymer, biological tests were carried out using the indirect contact method. Performed tests revealed that material is not inducing any cytotoxic response and ensures an adequate cell proliferation, as illustrated in
Figure 4. Furthermore, the numbers obtained from the measurements with the “BioTek Synergy HTX” showed that the viability of mouse fibroblast cells L929 in the presence of extract from analyzed materials was 98%, which indicates high biocompatibility in vitro on material.
4. Conclusions
In this work, we proved that the two-stage enzymatic polycondensation of 1,4-butanediol, diethyl succinate and dimer linoleic diol enable the production of sustainable aliphatic polyester, poly(butylene succinate-co-dilinoleic succinate) PBS-DLS 70:30 with the Mn value of 25,000 g/mol. 1H NMR analysis also revealed that the obtained material possesses a desirable molar composition and chemical structure, which indicate the good process control. Focusing on the thermal properties, we found that the Xc, Tm and Tg of PBS-DLS copolyester decreased after incorporation of amorphous DLS soft sequences. Finally, biocompatibility evaluations, conducted using L929 mouse fibroblast cells, highlighted the excellent in vitro biocompatibility of PBS-DLS copolyester, which is very beneficial for developing an eco-friendly approach and increases the range of potential applicability of these materials, for example, in biomedical applications.