Abstract
Microbial contamination represents an undesirable event in various domains. Bioactive natural compounds possess plenty of health benefits, including antimicrobial, antifungal and antioxidative activity; however, they are chemically unstable and susceptible to oxidative degradation. In this context, encapsulation or immobilization methods play a key role in enhancing efficiency. Therefore, in this paper are presented some results regarding the development of antimicrobial polymeric materials using surface-modification and emulsion-stabilization approaches. Two polymeric substrates, one biodegradable, poly(lactic acid), and one non-biodegradable polyethylene, functionalized by γ-irradiation have been modified with different active compounds in order to obtain bioactive food packaging materials. The bioactive agents (clove essential oil and argan vegetal oil) were incorporated into a biopolymer matrix (chitosan) then immobilized on the surface of the functionalized substrates by a wet-treatment involving carbodiimide chemistry. The resulted materials were physico-chemically characterized in order to evaluate the molecular interactions between the natural bioactive compounds and polymeric matrix, the stability of the immobilized surface layer, and their barrier properties. Antimicrobial and antioxidant activities were also evaluated. Moreover, the surface biofunctionalized polymeric substrates were tested as potential packaging materials for cheese preservation. The obtained materials have demonstrated improved barrier properties, good antioxidant and antimicrobial properties, which lead to a delay of the tested food spoilage.
1. Introduction
Microbial contamination represents an undesirable event in various domains, such as in biomedicine [1], food preservation [2], and cosmetics [3]. Microbial adhesion and growth on medical devices and implants can cause serious complications to human health, being a source of severe nosocomial infections [4]. The continuing emergence, development, and spread of pathogenic organisms that are resistant to antimicrobials constitute an increasing global concern [5]. The demand for new antimicrobial products is increasing due to the reduction in the effectiveness of existing industrial products that lead to continuous spread of infections. It is, however, extremely difficult to develop a universal product that could regulate or stop the entire infectious occurrence [6]. In the field of food preservation, substantial research on the development of innovative food packaging materials has been carried out aiming to combat pathogens, to reduce spoilage and waste, to optimize process efficiency, to decrease the need for chemical preservatives, improving the functionality, and the nutritional and sensorial properties of food. Despite efforts and improvements in the food production industry, foodborne pathogens still cause a number of illness outbreaks yearly all over the world [7].
Plants remain an unique and underexploited resource of bioactive compounds. Existing ethnobotanical traditions can be used to guide future research efforts and narrow down the search for the most suitable biocomponents to be used in modern antimicrobial materials [8]. The application of bioactive compounds in native form is very limited in food and drug formulations due to their poor bioavailability, low solubility, and fast release from traditional carriers [9]. In order to use these substances in specific applications, protection and stabilization in formulations, as well as controlled release, become compulsory.
In most circumstances, food-packaging materials are polymer-based. However, this has limitations, since plastics are semi-permeable to gases and can undesirably affect food and drink quality over relatively short time periods. Barrier properties of plastic materials can be improved by coatings [10,11] or through the inclusion of fillers (e.g., nanoparticles) within the polymer matrix [12]. Because of the inert nature of most commercial polymers, they must be subjected to surface functionalization before the attachment of bioactive compounds [13]. In this context, high-energy radiation appears as useful and suitable techniques for functionalization of both synthetic and natural polymers since they do not require the use of toxic chemicals and there is no waste production involved. In the present study, we focused on the surface functionalization of two inert polymers (poly(lactic acid) and polyethylene) and aimed to impart bioactive functionalities to the selected polymeric materials. In our research, chitosan (CS) was chosen as the incorporating matrix for essential and vegetal oils and further as bioactive coating for the surface functionalized polymeric substrates. CS belongs to the class of natural polysaccharides, has film forming ability, oxygen barrier property, and antimicrobial activity (against various pathogenic and spoilage micro-organisms) [14,15]. However, the antimicrobial activity of chitosan depends on its molecular weight, the degree of deacetylation and its chemical degradation [16], hence the incorporation of bioactive natural substances may lead to the improvement of its antimicrobial functions.
2. Experiments
Polymeric films, poly(lactic acid) (PLA) (2002D type, NatureWorks LLC, Minnesota, USA) and polyethylene (PE) (composed of two parts UV-treated LDPE and one part HDPE, provided by SC Loracom SRL, Roman, Romania) were γ-irradiated using an irradiation machine (GAMMATOR M-85) equipped with 137Cs source. The irradiation doses for polyethylene were 20, 30, and 50 kGy, while PLA was exposed to 10, 20, and 30 kGy, at a dose rate of 0.4 kGy h−1, in air, at room temperature. After γ-irradiation, the polymeric films were removed from the treatment chamber and surface coated by immersion for 12 h at 4 °C into a 2 wt% chitosan solution (containing 5% acetic acid) or oil-loaded chitosan emulsions. Food grade vegetal oils (clove essential oil—CEO, and cold pressed argan oil—CAO) were incorporated in a ratio of 0.75 mL per gram of chitosan (Sigma Aldrich, Munich, Germany), and Tween 80 (Sigma Aldrich, Munich, Germany) (0.125 g/g chitosan) was added as an emulsifying agent. The vegetal oil-added coating solution was homogenized with an ultrasonic processor UP50H (Hielscher—Ultrasound Technology, Teltow, Germany) using a power of 50 W at 30 kHz. The films modified with chitosan or chitosan-based emulsions were excessively rinsed first with 1% aqueous acetic acid solution and secondly with water, and further vacuum dried at 40 °C. For covalent bonding of chitosan coating onto PLA surface, the hydroxyl groups of chitosan were previously activated with 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) coupling agent. The samples thus prepared were kept in a desiccator until further analyses were performed.
The samples were characterized by FTIR spectroscopy at 4 cm−1 resolution with 64 scans (VERTEX 70 spectrometer, Bruker Optics, Ettlingen, Germany) and in terms of barrier properties by using a manometric gas permeation tester (Lyssy L100-5000, Systech Illinois, Johnsburg, IL, USA). Antioxidant activity was evaluated by DPPH (1,1-diphenyl-2-picryl-hydrazyl) assay as described in previously reported papers [11]. Antibacterial tests were performed using specific bacteria usually tested for food products both of animal and non-animal origin, namely Listeria spp. and Escherichia coli, by colony counting method [10]. The migration of the active components from the oil-loaded chitosan coatings was investigated by a total immersion migration test (EC, 1997) [17] using 50% aqueous ethanolic solution as a food simulant (D1) [18].
Food shelf life testing: The PLA and PE-surface functionalized films with oil-loaded chitosan coatings were tested as active materials for white cheese. Firstly, was determined the total viable counts (TVC) for fresh white cheese at 30 °C (ISO 4833:2003; Plate Count Agar at 30 + 1 °C for 72 h in aerobic conditions). The number of those bacteria in fresh control sample was less than 10 CFU/g. Most often, the microbiological spoilage of white cheese during its storage is caused by yeast and mold. Therefore, in this study we monitored the growth of those groups of microorganisms in white cheese stored in refrigerator in contact with active polymeric films.
3. Results
3.1. FTIR Spectroscopy
In Figure 1, the FT-IR spectra of poly(lactic acid) (Figure 1a) and polyethylene (PE) (Figure 1b)-based samples surface functionalized by chitosan and oil-loaded-chitosan coatings are illustrated.
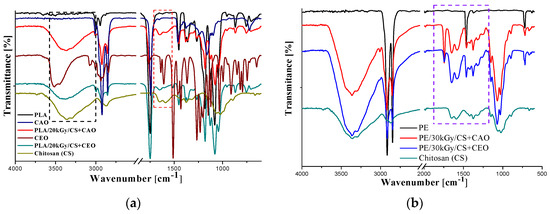
Figure 1.
FTIR spectra of neat, γirradiated and surface functionalized with oil-loaded chitosan coatings of (a) PLA- and (b) PE-based films.
The incorporation of vegetal oils induces significant changes in FTIR spectra of chitosan matrix. The band assigned to free OH bonds is found in the range of 3650–3590 cm−1 and the vibration band associated with the hydroxyl group involved in hydrogen bonding appear in 3600-3200 cm-1 wavenumbers domain. The FTIR spectrum ranging from 1400 to 400 cm-1 is considered the fingerprint region; the bands within this range are assigned to different vibrations such as C-H deformation and C-O valence vibrations from alcohols, ethers and esters. The representative bands in the FTIR spectrum of CEO (Figure 1a) are located at: 3511.3 cm−1 (phenolic νOH), 2937.6 cm−1 (aromatic νCH), 1511.2 cm−1 (aromatic νC-C), 1265.3 cm−1 (etheric νC-O) and at 1033 cm−1 (δ=C-H)[19]. The characteristic bands of both chitosan and CEO appear in the FTIR spectra of PLA after γ-irradiation and coating with CEO-loaded chitosan emulsion. The FTIR band at 1511 cm−1 assigned to νC-C from aromatic ring, found in CEO, is also evidenced in the PLA/20kGy/CS+CEO sample.
For argan oil (CAO) the IR spectrum (Figure 1a) reveals that: in the interval of 3100–2800 cm−1 the major absorption bands are located in the vicinity of frequencies 3006.9, 2923, 2853 cm−1. The band at 3006 cm−1 is specific to the methyl-oleate and the other absorptions are characteristic to the symmetrical and asymmetrical vibrations ν(C-H) of the CH2 and CH3 aliphatic groups from the alkyl rest of the triglycerides, which are found in large quantities in vegetal oils. The absorption at 1743 cm−1 is assigned to ν(C = O) from RC = OOR compounds, a feature of the oils with a high content in saturated fatty acids and short carbohydrate chain. The spectral band near to 1654 cm−1 corresponds to the double C = C link and may be correlated with the content of polyunsaturated fatty acids in the oil. At 1461 cm−1 a band associated to the vibrations of deformation δ(CH) is noticed. Another two bands are observed at 1376 and 1237 cm−1; the first band corresponds to the deformation vibration in the phase of methylene group, while the second band corresponds to the deformation vibration in the plan of the group =CH, from the double unconjugated cis-links [20]. By CAO loading CS emulsion, a new band appears in the FTIR spectra of PE/30kGy/CS+CAO at 1744.5 cm−1 (when compared with γ-irradiated PE coated only with chitosan) that is assigned to ν(C = O) from RC = OOR functional group. In the case of PLA substrate surface modified with CAO-loaded chitosan coating, some characteristic bands of PLA and chitosan overlaps the argan oil vibration bands. The FTIR spectrum of PLA/20kGy/CS+CAO sample reveals some characteristic bands (or shoulder peak) for argan oil, namely at 1462 and 1377 cm−1, attributed to vibrations of deformation in-plane and in-phase of methylene group.
3.2. Barrier Properties, Antioxidant/Antimicrobial Activities, and Migration in Food Simulant
The barrier properties of PLA and PE surface functionalized samples were estimated by measuring the gas transmission rate of oxygen (OTR) at 23 °C in relative dry state. Surface immobilization of chitosan and oil-loaded chitosan emulsions onto γ-irradiated poly(lactic acid) and polyethylene substrates leads to a significant decrease of OTR (Table 1). The antioxidant activity of the polymeric substrates functionalized by oil-loaded chitosan coatings was determined by measuring the capacity of each sample to scavenge the DPPH free radicals. Gamma irradiated PLA and PE modified only with chitosan presents only a slight antioxidant activity, while the substrates functionalized with vegetal oil-loaded chitosan emulsions exhibit enhanced radical scavenging activity (RSA) (Table 1). The samples functionalized with CEO-loaded chitosan manifest higher RSA compared to CAO-loaded coatings, as revealed by the data listed in Table 1. The migration profiles of CEO in food simulant D1 show a burst release of ~30% in the first hours, followed by a gradual but fast release in the first 4–5 days and then a slower release up to 2 weeks. The calculated kinetic parameters indicate a migration behavior with some tendency towards Fickian diffusion.

Table 1.
Oxygen permeability, antioxidant activity and bacterial inhibition (for a Gram-negative bacteria, E. coli, and a Gram-positive bacteria, Listeria monocytogenes) for PLA and PE functionalized surfaces with oil-loaded chitosan coatings.
3.3. Testing the Polymeric Substrates as Active-Food Packaging for Fresh White Cheese
The population dynamics of spoilage-related microorganisms (Total Viable Counts—TVC) of white cheese are described in Figure 2. It can be noticed that the TVC of white cheese stored in refrigerator in contact with traditional packaging material (control) were significantly higher than in PLA and PE substrates surface modified with chitosan and bioactive oil-loaded CS coating.
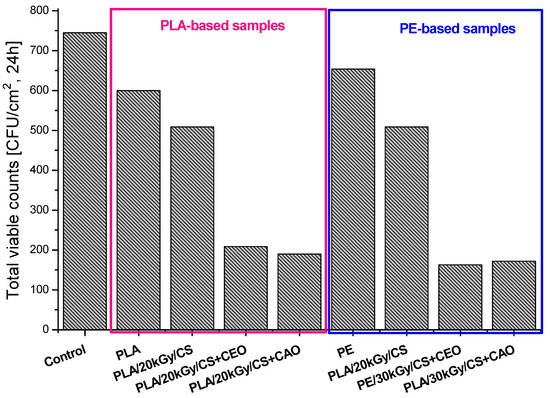
Figure 2.
Variation in time of Total Viable Counts for white cheese packed in poly(lactic acid) and polyethylene modified substrates with chitosan and oil-loaded chitosan emulsions.
4. Discussion
FTIR spectroscopy analysis of the samples had revealed that γ-irradiation of both polymeric substrates create reactive sites onto the surface that facilitates the attachment of biopolymeric formulations (chitosan or oil-loaded CS emulsions). Oil-loaded chitosan coatings are characterized mainly by strong hydrogen bond interactions (highlighted by the O-H band shifts to lower frequency). The FTIR spectroscopy demonstrated both clove and argan oil incorporation into chitosan matrix and the surface immobilization of the chitosan-oil mixture onto γ-irradiated PE and PLA substrates.
In dry conditions, the coated films were significantly less gas (oxygen) permeable than PLA and PE substrates. However, we must take into account the hygroscopic nature of chitosan, meaning that a rise in the relative humidity is possible to determine a decrease in its barrier properties. Moreover, the oxygen barrier properties are influenced by the thickness of the analyzed substrate, and this is one of the reasons that the OTR values for PLA-coated samples are lower than for PE-based samples (PLA films are 5 times thicker).
The antioxidant activity of the essential and vegetal oils is mainly dictated by their complex composition. In the case of CEO, the antioxidant activity is mainly attributed to eugenol, which is the major compound, and for CAO the antioxidant feature is dictated probably by the content in caffeic acid, vanillic acid, and ferulic acid. The migration of CEO active compounds into D1 food simulant was characterized by a prolonged and sustained release. This behaviour was determined by the incorporation of CEO in CS and subsequently the physico-chemical interactions at the oil-biopolymer interphase, which delayed the migration of active compounds. The migration behaviour of CAO could not be determined using D1 as food simulant, hence additional studies are planned.
The antibacterial tests show that the oil loading into chitosan coating leads to a slight decrease of CS antibacterial activity towards Listeria monocytogenes, which can be attributed to the interactions that take place between active components of oils and biopolymer (some functional groups of chitosan are hindered). Significantly lower values for TVC of spoilage-related microorganisms found in white cheese stored in contact with PE and PLA functionalized materials with oil-loaded CS coatings indicate a delay in food spoilage.
5. Conclusions
Surface functionalization of PLA and PE by gamma irradiation and active oil-loaded CS coatings improved their gas barrier properties, and imparted antioxidant and antimicrobial properties. The incorporation of active vegetal oils into chitosan coatings leads to a significant decrease of spoilage-related microorganisms of cheese when compared with the native polymeric substrates, PLA and PE, and to a sustained release of active compounds. Both clove essential oil and cold pressed argan oil proved to be valuable antimicrobial agents for delaying the spoilage of white cheese.
Author Contributions
E.S. and C.V. conceived and designed the experiments; E.S., R.P.D., M.B. performed the experiments; E.S., R.P.D. and M.B. analyzed the data; A.E. contributed with analysis tools; E.S. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Funding
The financial support of this work provided by a grant of the Romanian Ministry of Education and Research, CNCS—UEFISCDI, project number PN-III-P1-1.1-PD-2019-1101, number PD 31/2020, within PNCDI III, and European Social Fund for Regional Development, Competitiveness Operational Programme Axis 1—Project “Petru Poni Institute of Macromolecular Chemistry—Interdisciplinary Pol for Smart Specialization through Research and Innovation and Technology Transfer in Bio(nano)polymeric Materials and (Eco)Technology”, InoMatPol (ID P_36_570, Contract 142/10.10.2016, cod MySMIS: 107464) is gratefully acknowledged.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The support from Traian Zaharescu in gamma irradiation is gratefully acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PLA | Poly(lactic acid) |
| PE | Polyethylene |
| CEO | Clove essential oil |
| CAO | Cold pressed argan oil |
| CS | Chitosan |
| TVC | Total viable counts |
References
- Messina, G.; Ceriale, E.; Lenzi, D.; Burgassi, S.; Azzolini, E.; Manzi, P. Environmental Contaminants in Hospital Settings and Progress in Disinfecting Techniques. BioMed Res. Int. 2013, 2013, 429780. [Google Scholar] [CrossRef] [PubMed]
- Gizaw, Z. Public health risks related to food safety issues in the food market: A systematic literature review. Environ. Health Prev. Med. 2019, 24, 68. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Seok, Y.S.; Cho, T.J.; Rhee, M.S. Risk factors influencing contamination of customized cosmetics made on-the-spot: Evidence from the national pilot project for public health. Sci. Rep. 2020, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Klevens, R.M.; Edwards, J.R.; Richards, C.L.; Horan, T.C.; Gaynes, R.P.; Pollock, D.A.; Cardo, D.M. Estimating Health Care-Associated Infections and Deaths in U.S. Hospitals, 2002. Public Health Rep. 2007, 122, 160–166. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Antimicrobial Resistance in the Food Chain. Available online: https://www.who.int/foodsafety/areas_work/antimicrobial-resistance/en/ (accessed on 16 October 2020).
- Tiwari, A. Handbook of Antimicrobial Coatings; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- World Health Organization. Food Saftey. Available online: https://www.who.int/news-room/fact-sheets/detail/food-safety (accessed on 16 October 2020).
- Quave, C.L. Antibiotics from Nature: Traditional Medicine as a Source of New Solutions for Combating Antimicrobial Resistance. R&D Innovation 2016. Available online: http://resistancecontrol.info/rd-innovation/antibioticsfrom-nature-traditional-medicine-as-a-source-of-new-solutions-for-combating-antimicrobial-resistance/ (accessed on 8 October 2020).
- Shishir, M.R.I.; Xie, L.; Sun, C.; Zheng, X.; Chen, W. Advances in micro and nano-encapsulation of bioactive compounds using biopolymer and lipid-based transporters. Trends Food Sci. Technol. 2018, 78, 34–60. [Google Scholar] [CrossRef]
- Stoleru, E.; Dumitriu, R.P.; Munteanu, B.S.; Zaharescu, T.; Tănase, E.E.; Mitelut, A.; Ailiesei, G.-L.; Vasile, C. Novel procedure to enhance PLA surface properties by chitosan irreversible immobilization. Appl. Surf. Sci. 2016, 367, 407–417. [Google Scholar] [CrossRef]
- Stoleru, E.; Zaharescu, T.; Hitruc, E.G.; Vesel, A.; Ioanid, E.G.; Coroaba, A.; Safrany, A.; Pricope, G.; Lungu, M.; Schick, C.; et al. Lactoferrin-Immobilized Surfaces onto Functionalized PLA Assisted by the Gamma-Rays and Nitrogen Plasma to Create Materials with Multifunctional Properties. ACS Appl. Mater. Interfaces 2016, 8, 31902–31915. [Google Scholar] [CrossRef] [PubMed]
- Darie, R.N.; Pâslaru, E.; Sdrobis, A.; Pricope, G.M.; Hitruc, G.E.; Poiată, A.; Baklavaridis, A.; Vasile, C. Effect of nanoclay hydrophilicity on the poly(lactic acid)/clay nanocomposites properties. Ind. Eng. Chem. Res. 2014, 53, 7877–7890. [Google Scholar] [CrossRef]
- Goddard, J.; Hotchkiss, J. Polymer surface modification for the attachment of bioactive compounds. Prog. Polym. Sci. 2007, 32, 698–725. [Google Scholar] [CrossRef]
- Tokura, S.; Ueno, K.; Miyazaki, S.; Nishi, N. Molecular weight dependent antimicrobial activity by Chitosan. Macromol. Symp. 1997, 120, 1–9. [Google Scholar] [CrossRef]
- Tsai, G.-J.; Su, W.-H.; Chen, H.-C.; Pan, C.-L. Antimicrobial activity of shrimp chitin and chitosan from different treatments and applications of fish preservation. Fish. Sci. 2002, 68, 170–177. [Google Scholar] [CrossRef]
- Omura, Y.; Shigemoto, M.; Akiyama, T.; Saimoto, H.; Shigemasa, Y.; Nakamura, I.; Tsuchido, T. Antimicrobial Activity of Chitosan with Different Degrees of Acetylation and Molecular Weights. Biocontrol Sci. 2003, 8, 25–30. [Google Scholar] [CrossRef]
- European Commission. Commission directive 97/48/EC of 29 July 1997 amending for the second time council directive 82/711/EEC laying down the basic rules necessary for testing migration of the constituents of plastic materials and articles intended to come into contact with foodstuffs (97/48/EC). J. Eur. Commun. 1997, 222, 210–215. [Google Scholar]
- McClements, D.J.; Decker, E.A. Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Sadanand Kadam, S.; Waghmare Jyotsna, S. Identification of major volatile (essential oil) constituents of “carrom seeds” and “clove buds”. IJSRR 2014, 3, 85–94. [Google Scholar]
- Alexa, E.; Dragomirescu, A.; Pop, G.; Jianu, C.; Dragoş, D. The use of FT-IR spectroscopy in the identification of vegetable oils adulteration. J. Food Agric. Environ. 2009, 7, 20–24. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).