Abstract
Cat’s claw and aloe vera gel contain active compounds and could be used to enhance the properties of wound dressings. Cat’s claw is known for its anti-inflammatory, anti-arthritic and anti-asthmatic properties; and aloe vera is commonly used for wound healing and skin hydration. In this study, we elaborated microparticles from an emulsion made of alginate solutions with aloe vera gel or cat’s claw extract with ultrasound and tri-dimensional membranes obtained by solvent evaporation. The 27 to 33 µm-thick membranes showed a porous surface on scanning electron microscopy (SEM); the contact angle of water on the membranes increased in hydrophilicity due to the use of aloe vera gel. Furthermore, the presence of aloe vera also improved water absorption in an acetate buffer (pH 5.5) at 37.5 °C. Finally, the presence of cat’s claw extract in the microparticles significantly enhanced radical scavenging in the 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), ABTS, decoloration assay, in comparison to tri-dimensional alginate membranes with no active compounds. Alginate films with cat’s claw extract or aloe vera gel could be used as wound dressings materials.
1. Introduction
The use of wound dressings is common practice when there is a skin injury. Wound dressings help in the healing process and their role is to provide an adequate environment as they are in direct contact with the wound, and this includes the need of reducing the risk of infection while minimizing loss of function of the skin [1]. Wound dressings can be classified into traditional and polymeric dressings. Traditional materials, such as gauze or bandages, present certain risks since they do not provide a suitable humid environment and are prone to adhere to the wound, which can cause a prolongation of the healing process, an aggravation of the wound condition, and, in severe cases, even hospitalization [1,2]. On the other hand, polymeric dressings (natural or synthetic), although they present various improvements compared to traditional materials, as they can be used in different types of acute and chronic wound and demonstrate good permeability to water vapor, they also have disadvantages, such as being opaque and poor absorbents of wound fluid [1,2].
Among the polymeric materials, the use of biopolymers—such as alginate—is of great interest. Alginate is a linear polysaccharide that is biocompatible, bio-degradable and known for its ability to form films. Previous studies [3] consider that this polymer can be used together with bioactive substances, such as aloe vera or a plant extract, to optimize its therapeutic properties.
Aloe vera (Aloe barbadensis) has been used in traditional medicine and therefore it is a highly studied plant due to its biological activities. Aloe contains a gelatinous matrix that contains between 98.5% and 99.5% of water and a solid phase with more than 75 active components such as polysaccharides, enzymes, amino acids and vitamins [4]. Previous studies [5,6] consider that acemannan, a polysaccharide, is the major solid component of the plant. Aloe vera gel is anti-inflammatory, antibacterial and accelerates the healing of small wounds and burns [3,7].
Cat’s claw (Uncaria tomentosa, UT) is a popular medicinal plant used as an anti-inflammatory, immune system stimulator, anti-antioxidant and antiviral, as well as a treatment for cardiovascular diseases [8], tumors [9], etc. Most of the studies on cat’s claw are centered on its alkaloids [8,10] (tetracyclic and pentacyclic oxindole, indole, and triterpenoid), especially because of its abundancy. Cat’s claw also has a great number of polyphenolic compounds that can be classified into the following groups for better comprehension: hydroxybenzoic acids (including gallic, salicylic and vanillic acids), hydroxy-cinnamic acids (caffeic, p-coumaric, ferulic and isoferulic acids), flavonoids ((+)-catechin and (−)-epicatechin monomer), condensed tannins (several procyanidins and propelargonidin) and flava-lignans (four cinchonain) [11].
The main contribution of the polyphenolic compounds to the properties of cat’s claw is their antioxidant activity. Structurally, polyphenols possess numerous conjugated systems and hydroxyls that can react with radical species and deactivate them—a process called radical scavenging. However, polyphenolic compounds also play a role in the modulation of pathways involved in chronic inflammation [12]. Some studies also show positive results when evaluating the antimicrobial activity of rich polyphenolic extracts over Staphylococcus aureus and the in vitro cytotoxic activity of such extracts over colon adenocarcinoma cell lines [13,14].
2. Experimental Part
2.1. Materials
Alginate (AL) and the non-ionic surfactant poloxamer 407 were purchased from Sigma-Aldrich (St. Louis, Missouri). Cat’s claw bark Naturandes® and aloe vera leaves were obtained from a local market in Lima.
2.2. Plant Extraction Process
The cat’s claw extract (UT) was obtained by ultrasonic bath for 20 min using ethanol 60% (v/v) as solvent (three times). The liquid phase was centrifuged to separate the fine particles, then the ethanol was removed at low pressure and finally the extract was lyophilized to obtain a powder.
Aloe vera leaves were washed and the gel was separated from the skin and washed again with plenty of water. Next, the gel was cut into small pieces, blended and filtered to separate the fibers. The liquid was centrifuged and stabilized at 65 °C for 15 min. The gel was stored at 4 °C.
2.3. Microparticles and Film Formation
A 1% (w/v) aqueous alginate solution was mixed with cat’s claw extract to a final concentration of 0.1% (w/v) (AL-UT). Surfactant (poloxamer 407) was added (0.1% or 0.5%, w/v) along with the organic solvent (hexane) in a 1:9 (v/v) ratio. The mixture was cooled for 15 min in an iced bath and then sonicated (Sonics, 20 kHz, 750 W, 40% amplitude) for 3 min. The emulsion obtained was poured on a Petri dish and left to dry at 50 °C for 24 h.
Aloe vera gel (AVG) with alginate films were prepared in the same manner as AL-UT films but in different concentrations: AVG 10% (v/v) or AVG 25% (v/v).
Additionally, a control film—with no UT or AVG—was obtained by the same procedure with poloxamer 0.5% (w/v). Finally, all the films obtained were crosslinked with 1% calcium chloride solution for 5 min, rinsed with water and left to dry at room temperature.
2.4. Characterization of Films
2.4.1. Thickness and Morphological Analysis
Thickness of films was measured with a micrometer (Mitutoyo, Japan). Six thickness measurements were taken along the film, including the center.
Scanning electron microscopy (SEM, Delong America—Canada, model LVEM5) was used to study the surface of films. SEM images were taken at an accelerating voltage of 5 kV.
2.4.2. Swelling Behavior
This experiment was performed using a 2 × 2 cm slice of the films. Pre-weighted films were immersed in an acetate buffer (pH 5.5) at 37.5 °C. After a period of time, the hydrated films were collected, and the excess of buffer was removed with a filter paper and weighted. Weights were measured after 20, 40, 60, 180, 300 and 1440 min. The swelling was determined according to Equation (1):
where mswell represents the mass of the swollen film and mdry corresponds to the mass of the dry piece of film. Three samples were evaluated for each type of film.
2.4.3. Wettability
Contact angle of films was measured using a Ramé-Hart goniometer, model 250 (Succasunna, New Jersey) and distilled water as solvent (5 µL drop). The measurements were made at six different points on the surface of films; 50 measurements were collected every 0.005 s at each point. Drop Image software was used to collect the data.
2.4.4. Antioxidant Activity
The 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid), ABTS, radical cation decoloration assay was used to evaluate antioxidant activity. ABTS was dissolved in ethanol and a slice of film was used as sample. The absorbance was measured at 734 nm after 1, 5, 15, 30, 45 min. The inhibition was calculated with Equation (2):
where Acontrol is the absorbance of ABTS solution alone and Asample represents the absorbance of ABTS solution with the sample after a certain time.
3. Results
3.1. Physical Appearance, Thickness and Morphological Analysis
Figure 1 and Figure 2 show the physical appearance of films of alginate with cat’s claw extract (UT) and alginate with aloe vera gel (AVG), respectively; a control film (alginate and poloxamer only) is also shown. All membranes are transparent and, in the case of cat’s claw, the films have a brown color. On the other hand, AL-AVG films are similar in comparison to the control film.
Figure 1.
Physical appearance of alginate with Uncaria tomentosa (UT) extract powder: (a) Alginate-Poloxamer 0.5% (control); (b) Alginate-Poloxamer 0.1%-UT0.1%; (c) Alginate-Poloxamer 0.5%-UT0.1%.
Figure 2.
Physical appearance of alginate with aloe vera gel: (a) Alginate-Poloxamer 0.5% (control); (b) Alginate-Poloxamer 0.5%-aloe vera gel (AVG)10%; (c) Alginate-Poloxamer 0.5%-AVG25%.
Figure 3 shows the thicknesses of alginate membranes, UT and AVG, which are between 27 and 33 µm. The control film’s thickness was 43 µm. The addition of cat’s claw or aloe vera gel decreased the film’s thicknesses. Moreover, the increase of aloe vera gel content reduced the thickness even more.
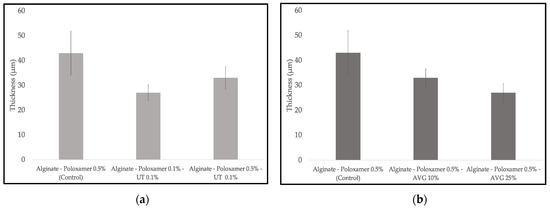
Figure 3.
Average thickness of alginate films with: (a) cat’s claw extract; (b) aloe vera.
The surface topography of alginate membranes with UT and with AVG was analyzed with SEM (Figure 4 and Figure 5). A membrane prepared with alginate solution (no emulsion) showed a smooth surface with no cracks (data not shown), while films made with microparticles presented a porous surface. Films of alginate with cat’s claw produced rough surfaces, which was also observed in control films of alginate and poloxamer (Figure 4a).
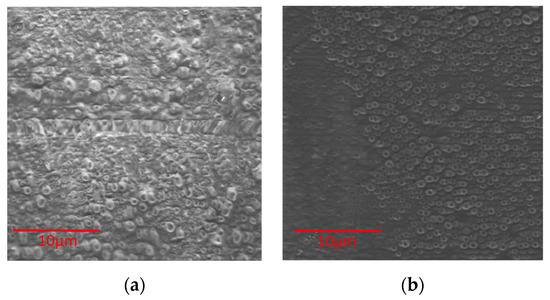
Figure 4.
SEM images of alginate with UT extract powder: (a) Alginate-Poloxamer 0.5% (control); (b) Alginate-Poloxamer 0.5%-UT0.1%.
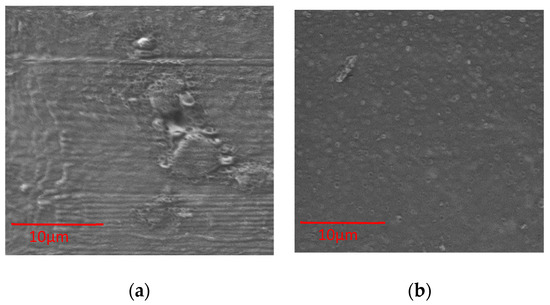
Figure 5.
SEM images of alginate with AVG: (a) Alginate-Poloxamer 0.5%-AVG10%; (b) Alginate-Poloxamer 0.5%-AVG25%.
3.2. Swelling Behavior
Figure 6 shows the swelling behavior of alginate with UT and AVG membranes in acetate buffer at 37.5 °C and pH 5.5 simulating skin. In both cases a control film (alginate-poloxamer 0.5%) was also studied. The average swelling behavior of alginate with cat’s claw films increased slightly compared to the control film; in the case of alginate with aloe vera films, the swelling was greater than in the control and it increased with the concentration of aloe vera gel. However, the alginate-AVG25% started to dissolve in the buffer after 3 h.
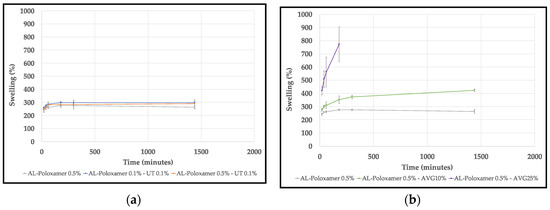
Figure 6.
Swelling behavior of: (a) alginate with UT extract powder films; (b) alginate with AVG films.
3.3. Wettability
Wettability was evaluated by the contact angle of a water drop which showed the hydrophilic/hydrophobic character of the films. Figure 7 shows the contact angles on AL-UT and AL-AVG films. The values on the AL-UT and control films are similar. In the case of the AVG, the film with 10% (v/v) of the gel has a similar contact angle to the control, but when AVG was increased to 25% the hydrophilicity of the film increased sharply and the water drop was absorbed, changing its contact angle from 53° to 4° within seconds.
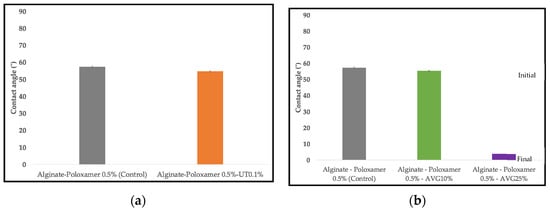
Figure 7.
Wettability of: (a) alginate with UT extract powder films; (b) Alginate with AVG films.
3.4. Antioxidant Activity
Figure 8 shows the antioxidant activity of the alginate films with the natural compounds. The film with UT shows an excellent antioxidant activity with almost 100% of inhibition after 15 min compared with the control film–alginate and poloxamer only–which has less than 5% of inhibition even after 45 min. On the other hand, the film with AVG 10% has a similar activity to the control film. The AL-AVG25% film could not be evaluated because it dissolved in the solvent.
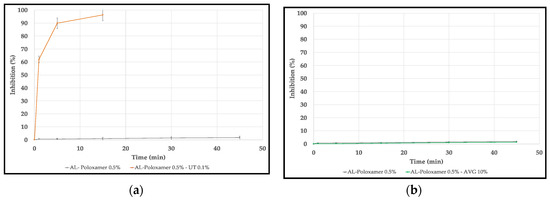
Figure 8.
Antioxidant activity of: (a) alginate with UT extract powder films; (b) Alginate with aloe vera gel films.
4. Discussion
4.1. Physical Appearance, Thickness and Morphological Analysis
All films were transparent and this is an important characteristic for wound dressings for monitoring injuries without removing the film [3]. The brown color of AL-UT films is due to the components of the cat’s claw extract. The increase of AVG in films led to a decrease of the film thickness, which is due to more water content from the gel during preparation; this was observed also by Pereira et al. [3].
SEM analysis shows a porous surface of the films that contained the active compounds, due to the formation of dispersed microparticles prior to the formation of the film. Similar results have been reported by Shankar et al. [15].
4.2. Swelling Behavior
The high values of water absorption of AL-AVG films could be explained by the hydrophilic character of the components of the gel, such as acemannan and other polysaccharides. Fluid absorption is important for wound treatment in order to maintain an adequate moisture environment on the injury [3].
4.3. Wettability
The contact angle analysis of the films showed a slight increase in the hydrophilicity of AL-UT films (3°), which could be due to the incorporation of phenolic compounds (rich in hydroxyl groups) in the cat’s claw extract. On the other hand, the noticeable increase in hydrophilicity of the AL-AVG films was due to the nature of the compounds present in AVG, as was described previously for the swelling behavior.
4.4. Antioxidant Activity
The high antioxidant activity of the AL-UT film can be explained by the presence of phenolic compounds. As explained above, ply-phenols can easily deactivate free radical species. In this case, when the ABTS radical approaches any of the polyphenolic compounds from the UT extract, it readily gains either a hydrogen atom or an electron from the hydroxyl groups in the antioxidants, which causes the solution to lose its characteristic blue color.
5. Conclusions
Tridimensional membranes made of alginate and cat’s claw extract (AL-UT), and of alginate and aloe vera gel (AL-AVG) were produced. The thickness of the membranes varied between 27 and 33 µm, all were uniform and transparent, and only the AL-UT films had a brownish color. Surface analysis via SEM shows that the films have porous surfaces.
The swelling study showed that AL-UT films remained intact in buffer acetate (pH 5.5) at 37.5 °C after 24 h, while AL-AVG25% film started to dissolve after 3 h. The surfaces of membranes of alginate with active compounds showed a hydrophilic character, and a higher content of AVG decreased the contact angle. The AL-UT film showed a high antioxidant activity with almost 98% inhibition after 15 min, while AVG did not show activity when compared to the control film. AL films functionalized with cat’s claw extract or aloe vera gel show important properties for potential use as wound dressing materials.
Author Contributions
S.K., A.D., M.E. and J.N. conceived and design the experiments; B.C.G. designed and performed the SEM analysis; M.E. and A.D. performed the experiments; all authors analyzed the data; M.E. and A.D. wrote the paper draft; J.N. and S.K. reviewed and edited the paper. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Concytec: Project N°151-2017-FONDECYT; and the Science Department-Chemistry Division, Pontificia Universidad Católica del Perú (PUCP).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, nor in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| UT: | Cat’s claw (Uncaria tomentosa) |
| AVG: | Aloe vera gel |
| AL: | Alginate |
| SEM: | Scanning electron microscopy |
| ABTS: | 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) |
References
- Kamoun, E.A.; Kenawy, E.-R.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Naseri-Nosar, M.; Ziora, Z.M. Wound dressings from naturally-occurring polymers: A review on homopolysaccharide-based composites. Carbohydr. Polym. 2018, 189, 379–398. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.; Mendes, A.; Bártolo, P. Alginate/Aloe Vera Hydrogel Films for Biomedical Applications. Procedia CIRP 2013, 5, 210–215. [Google Scholar] [CrossRef]
- Eshun, K.; He, Q. Aloe Vera: A Valuable Ingredient for the Food, Pharmaceutical and Cosmetic Industries—A Review. Crit. Rev. Food Sci. Nutr. 2004, 44, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Hamman, J. Composition and Applications of Aloe vera Leaf Gel. Molecules 2008, 13, 1599–1616. [Google Scholar] [CrossRef] [PubMed]
- Saberian, H.; Abbasi, S.; Hamidi-Esfahani, Z. Effect of pasteurization and storage on bioactive components of Aloe vera gel. Nutr. Food Sci. 2013, 43, 175–183. [Google Scholar] [CrossRef]
- Silva, S.S.; Popa, E.G.; Gomes, M.E.; Cerqueira, M.T.; Marques, A.P.; Caridade, S.G.; Teixeira, P.; Sousa, C.; Mano, J.F.; Reis, R.L. An investigation of the potential application of chitosan/aloe-based membranes for regenerative medicine. Acta Biomater. 2013, 9, 6790–6797. [Google Scholar] [CrossRef] [PubMed]
- Heitzman, M.E.; Neto, C.C.; Winiarz, E.; Vaisberg, A.J.; Hammond, G.B. Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae). Phytochemistry 2005, 66, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Pero, R.W.; Amiri, A.; Bryngelsson, C. Induction of apoptosis and inhibition of proliferation in human tumor cells treated with extracts of Uncaria tomentosa. Anticancer Res. 1998, 18, 3363–3368. [Google Scholar] [PubMed]
- Keplinger, K.; Laus, G.; Wurm, M.; Dierich, M.P.; Teppner, H. Uncaria tomentosa (Willd.) DC.—Ethnomedicinal use and new pharmacological, toxicological and botanical results. J. Ethnopharmacol. 1998, 64, 23–34. [Google Scholar] [CrossRef]
- Navarro-Hoyos, M.; Alvarado-Corella, D.; Moreira-Gonzalez, I.; Arnaez-Serrano, E.; Monagas-Juan, M. Polyphenolic Composition and Antioxidant Activity of Aqueous and Ethanolic Extracts from Uncaria tomentosa Bark and Leaves. Antioxidants 2018, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Aguilera, A.; Rull, A.; Rodríguez-Gallego, E.; Riera-Borrull, M.; Luciano-Mateo, F.; Camps, J.; Menéndez, J.A.; Joven, J. Mitochondrial Dysfunction: A Basic Mechanism in Inflammation-Related Non-Communicable Diseases and Therapeutic Opportunities. Mediat. Inflamm. 2013, 2013, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Lebrón-Aguilar, R.; Quintanilla-López, J.E.; Cueva, C.; Hevia, D.; Quesada, S.; Azofeifa, G.; Moreno-Arribas, M.; Monagas, M.; Bartolomé, B. Proanthocyanidin Characterization and Bioactivity of Extracts from Different Parts of Uncaria tomentosa L. (Cat’s Claw). Antioxidants 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Zamora, W.; Quesada, S.; Azofeifa, G.; Alvarado, D.; Monagas, M. Fractioning of Proanthocyanidins of Uncaria tomentosa. Composition and Structure-Bioactivity Relationship. Antioxidants 2017, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Kasapis, S.; Rhim, J.-W. Alginate-based nanocomposite films reinforced with halloysite nanotubes functionalized by alkali treatment and zinc oxide nanoparticles. Int. J. Biol. Macromol. 2018, 118, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).