Combinatory Action of Chitosan-Based Blended Films and Loaded Cajeput Oil against Staphylococcus aureus and Pseudomonas aeruginosa-Mediated Infections †
Abstract
:1. Introduction
2. Experiments
2.1. Materials
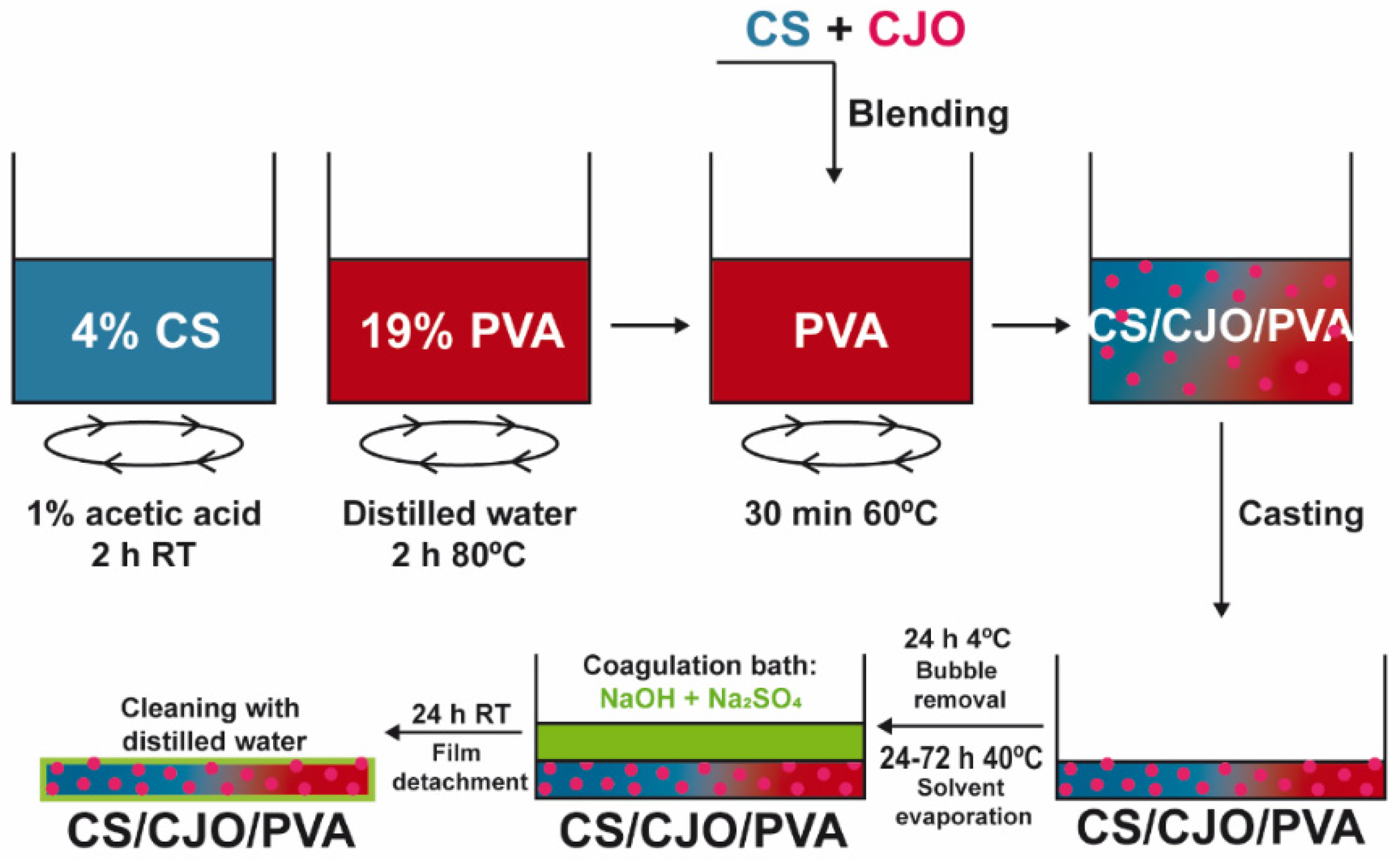
2.2. CS/CJO/PVA Film Production
2.3. Physical and Chemical Characterization
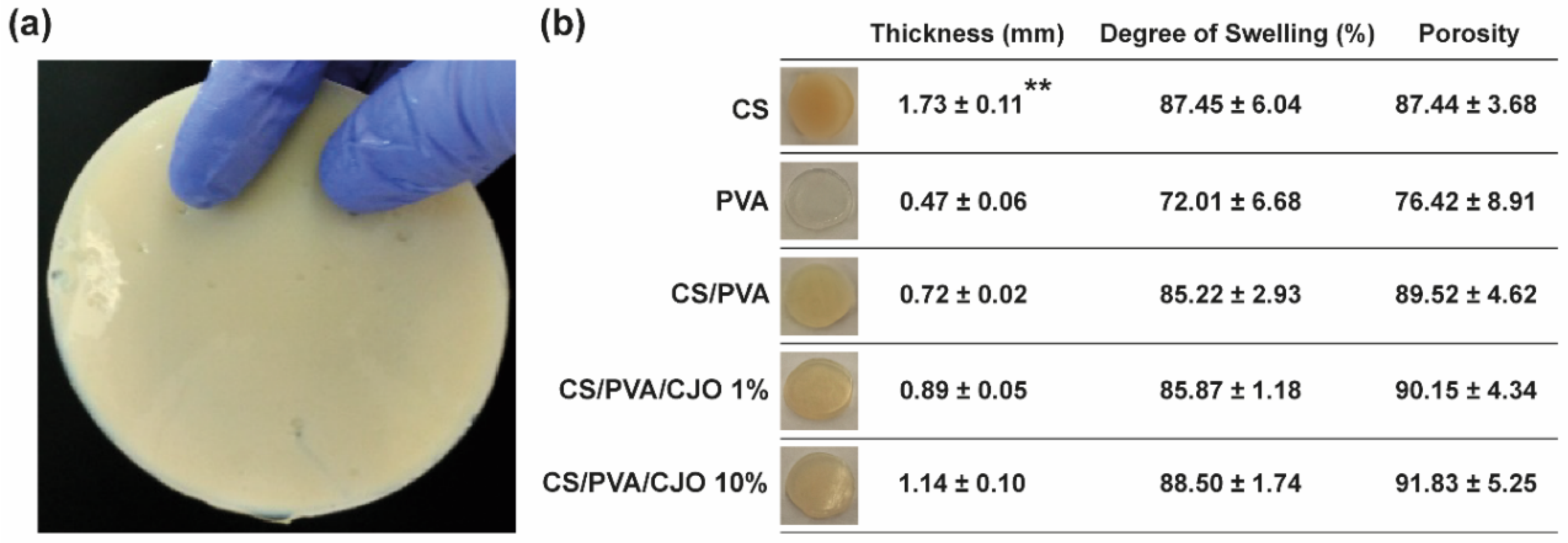
2.3.1. Macroscopic Assessment
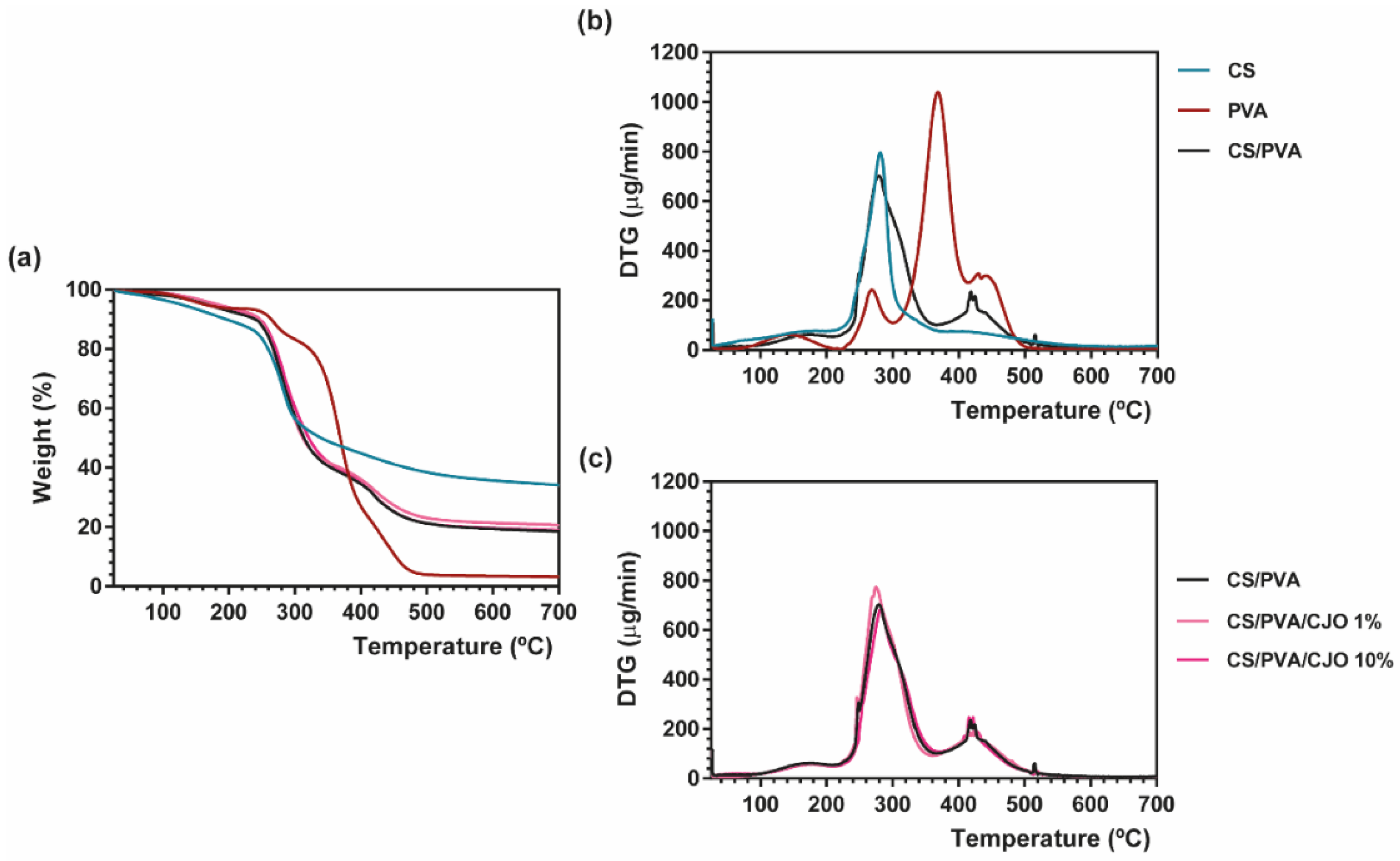
2.3.2. Thermal Properties
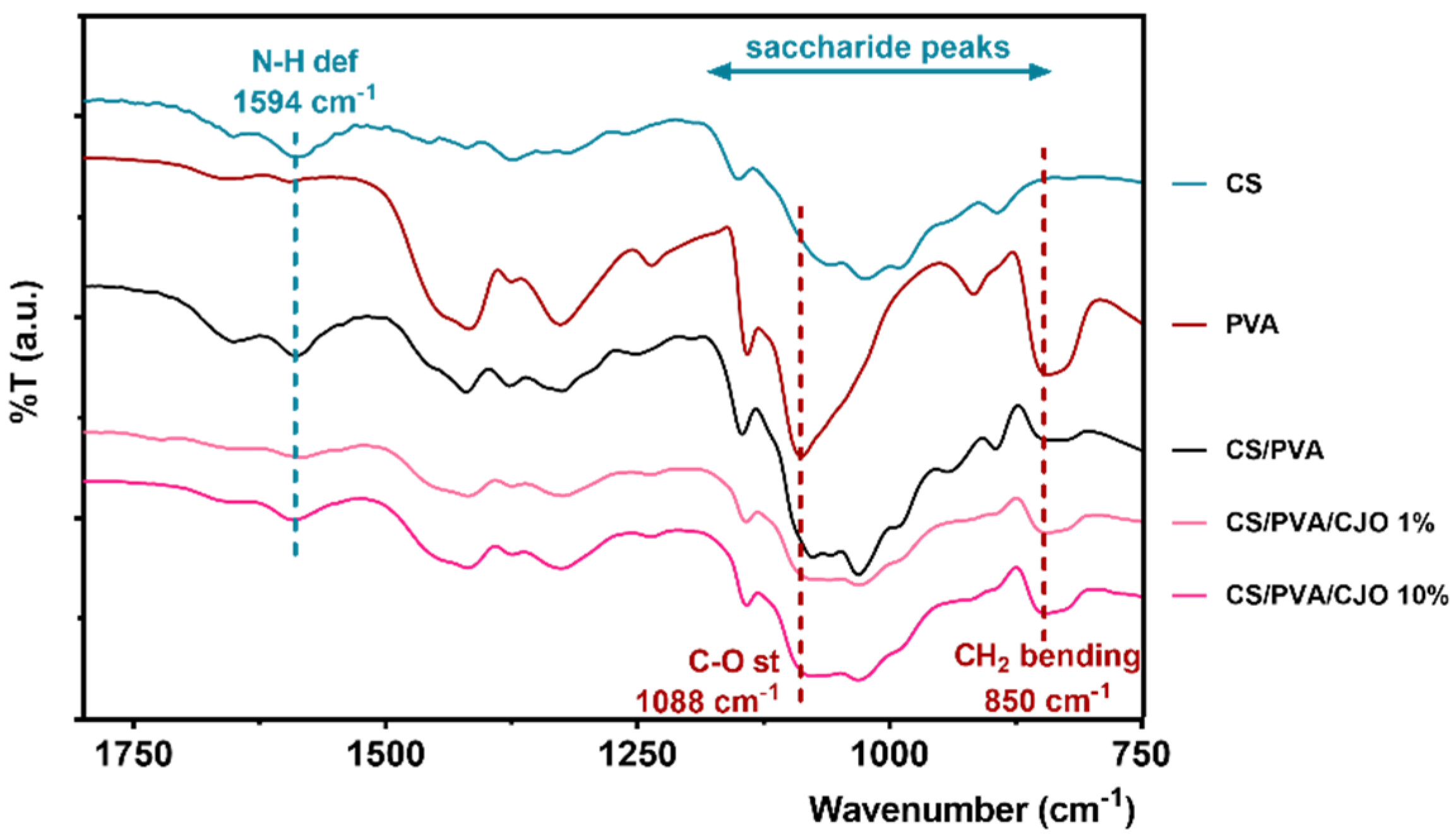
2.3.3. Chemical Composition
2.4. Antimicrobial Action
2.4.1. Time Kill Kinetics
2.4.2. Statistical Analysis
3. Results and Discussion
3.1. Macroscopic Assessment
3.2. Thermal Properties
3.3. Chemical Composition
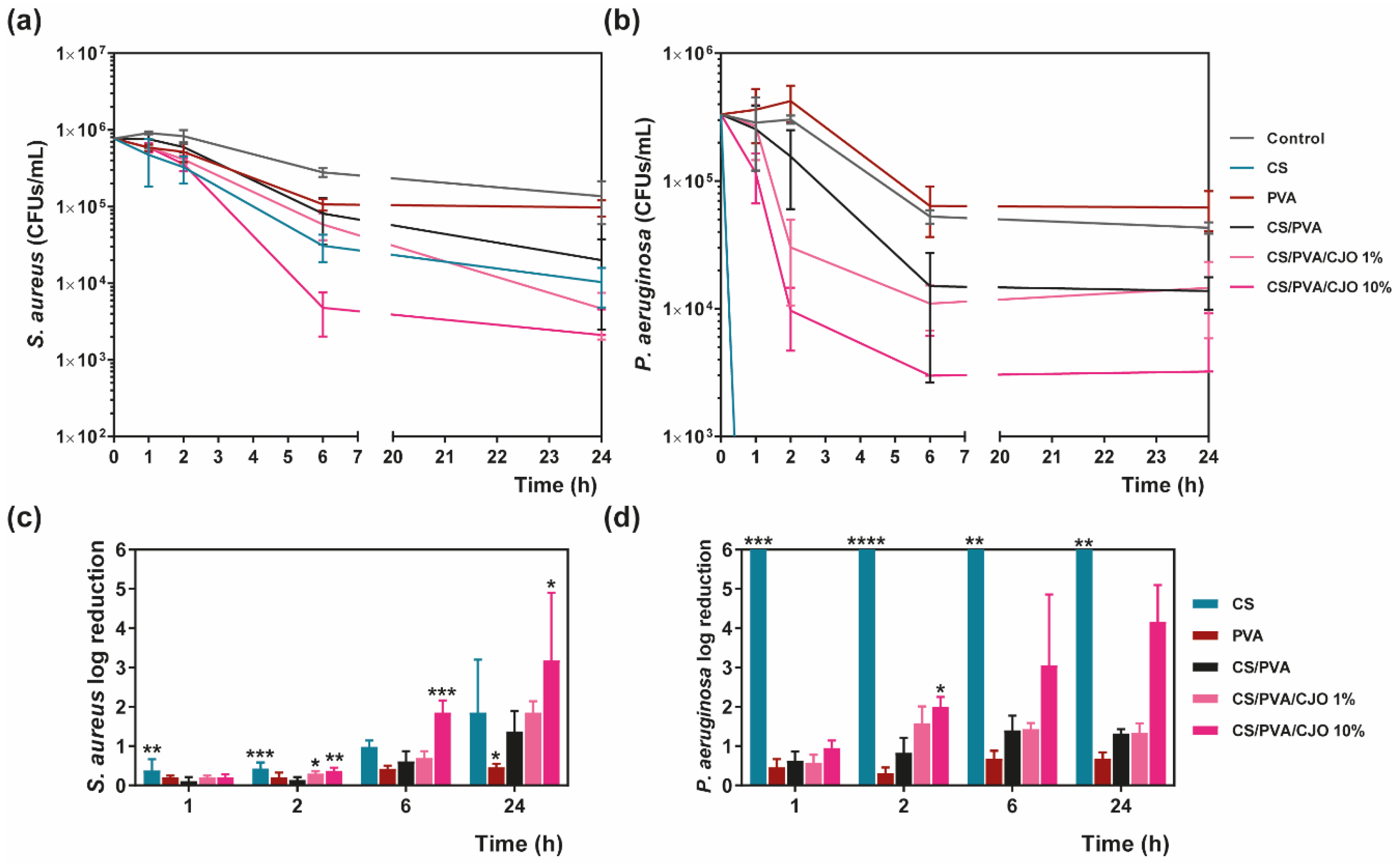
3.4. Antimicrobial Action
Time Kill Kinetics
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Noncommunicable Diseases. Available online: http://www.who.int/mediacentre/factsheets/fs355/en/ (assessed on 19 October 2020).
- Ramirez-Acuña, J.M.; Cardenas-Cadena, S.A.; Marquez-Salas, P.A.; Garza-Veloz, I.; Perez-Favila, A.; Cid-Baez, M.A.; Flores-Morales, V.; Martinez-Fierro, M.L. Diabetic Foot Ulcers: Current Advances in Antimicrobial Therapies and Emerging Treatments. Antibiotics 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.C.; Gonçalves, R.M.G.; Barbosa, M.A. Chitosan/Poly(γ-glutamic acid) Polyelectrolyte Complexes: From Self- Assembly to Application in Biomolecules Delivery and Regenerative Medicine. Res. Rev. J. Mater. Sci. 2016, 4, 12–36. [Google Scholar] [CrossRef]
- Kumar, M.N.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of Specialized Biomolecules against Gram-Positive and Gram-Negative Bacteria. Antibiot. 2020, 9, 314. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P.; Teixeira, M.A.; Tavares, T.D.; Homem, N.C.; Zille, A.; Amorim, M.T.P. Antimicrobial action and clotting time of thin, hydrated poly(vinyl alcohol)/cellulose acetate films functionalized with LL37 for prospective wound-healing applications. J. Appl. Polym. Sci. 2019, 137, 48626. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Koosha, M.; Hamedi, S. Intelligent Chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog. Org. Coat. 2019, 127, 338–347. [Google Scholar] [CrossRef]
- Merlusca, I.P.; Matiut, D.S.; Lisa, G.; Silion, M.; Gradinaru, L.; Oprea, S.; Popa, I.M. Preparation and characterization of chitosan–poly(vinyl alcohol)–neomycin sulfate films. Polym. Bull. 2017, 75, 3971–3986. [Google Scholar] [CrossRef]
- Wu, Y.; Ying, Y.; Liu, Y.; Zhang, H.; Huang, J. Preparation of chitosan/poly vinyl alcohol films and their inhibition of biofilm formation against Pseudomonas aeruginosa PAO1. Int. J. Biol. Macromol. 2018, 118, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Machado, C.; Teixeira, S.; Fonseca, L.; Abreu, M.; Carvalho, A.; Pereira, M.T.; Amaral, C.; Freitas, C.; Ferreira, L.; Neto, H.R.; et al. Evolutionary trends in bacteria isolated from moderate and severe diabetic foot infections in a Portuguese tertiary center. Diabetes Metab. Syndr. Clin. Res. Rev. 2020, 14, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Brugnerotto, J.; Lizardi, J.; Goycoolea, F.; Argüelles-Monal, W.; Desbrieres, J.; Rinaudo, M. An infrared investigation in relation with chitin and chitosan characterization. Polymer 2001, 42, 3569–3580. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Homem, N.C.; Teixeira, M.A.; Ribeiro, A.R.M.; Antunes, J.C.; Amorim, M.T.S.P. Physical, Thermal, and Antibacterial Effects of Active Essential Oils with Potential for Biomedical Applications Loaded onto Cellulose Acetate/Polycaprolactone Wet-Spun Microfibers. Biomolecules 2020, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Tin, S.; Sakharkar, K.R.; Lim, C.S.; Sakharkar, M.K. Activity of Chitosans in combination with antibiotics in Pseudomonas aeruginosa. Int. J. Biol. Sci. 2009, 5, 153–160. [Google Scholar] [CrossRef] [PubMed]
| EO | CS Solution | PVA Solution | Total %w/V | VTotal (mL) | CS/PVA Mass Ratios | ||||
|---|---|---|---|---|---|---|---|---|---|
| m (mg) | V (µL) | mCS (g) | V (mL) | mPVA (g) | V (mL) | ||||
| CS | - | - | 3.51 | 39 | - | - | 9% | 39 | 100/0 |
| PVA | - | - | - | - | 3.51 | 39 | 0/100 | ||
| CS/PVA | - | - | 1.053 | 26 | 2.457 | 13 | 30/70 | ||
| CS/PVA/CJO 1% | 35.1 | 39.2 | |||||||
| CS/PVA/CJO 10% | 351 | 392 | |||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antunes, J.C.; Tavares, T.D.; Homem, N.C.; Teixeira, M.A.; Amorim, M.T.P.; Felgueiras, H.P. Combinatory Action of Chitosan-Based Blended Films and Loaded Cajeput Oil against Staphylococcus aureus and Pseudomonas aeruginosa-Mediated Infections. Proceedings 2021, 69, 17. https://doi.org/10.3390/CGPM2020-07188
Antunes JC, Tavares TD, Homem NC, Teixeira MA, Amorim MTP, Felgueiras HP. Combinatory Action of Chitosan-Based Blended Films and Loaded Cajeput Oil against Staphylococcus aureus and Pseudomonas aeruginosa-Mediated Infections. Proceedings. 2021; 69(1):17. https://doi.org/10.3390/CGPM2020-07188
Chicago/Turabian StyleAntunes, Joana C., Tânia D. Tavares, Natália C. Homem, Marta A. Teixeira, M. Teresa P. Amorim, and Helena P. Felgueiras. 2021. "Combinatory Action of Chitosan-Based Blended Films and Loaded Cajeput Oil against Staphylococcus aureus and Pseudomonas aeruginosa-Mediated Infections" Proceedings 69, no. 1: 17. https://doi.org/10.3390/CGPM2020-07188
APA StyleAntunes, J. C., Tavares, T. D., Homem, N. C., Teixeira, M. A., Amorim, M. T. P., & Felgueiras, H. P. (2021). Combinatory Action of Chitosan-Based Blended Films and Loaded Cajeput Oil against Staphylococcus aureus and Pseudomonas aeruginosa-Mediated Infections. Proceedings, 69(1), 17. https://doi.org/10.3390/CGPM2020-07188








