Reduced Graphene Oxide Fibre Electrodes for Drug Sensing †
Abstract
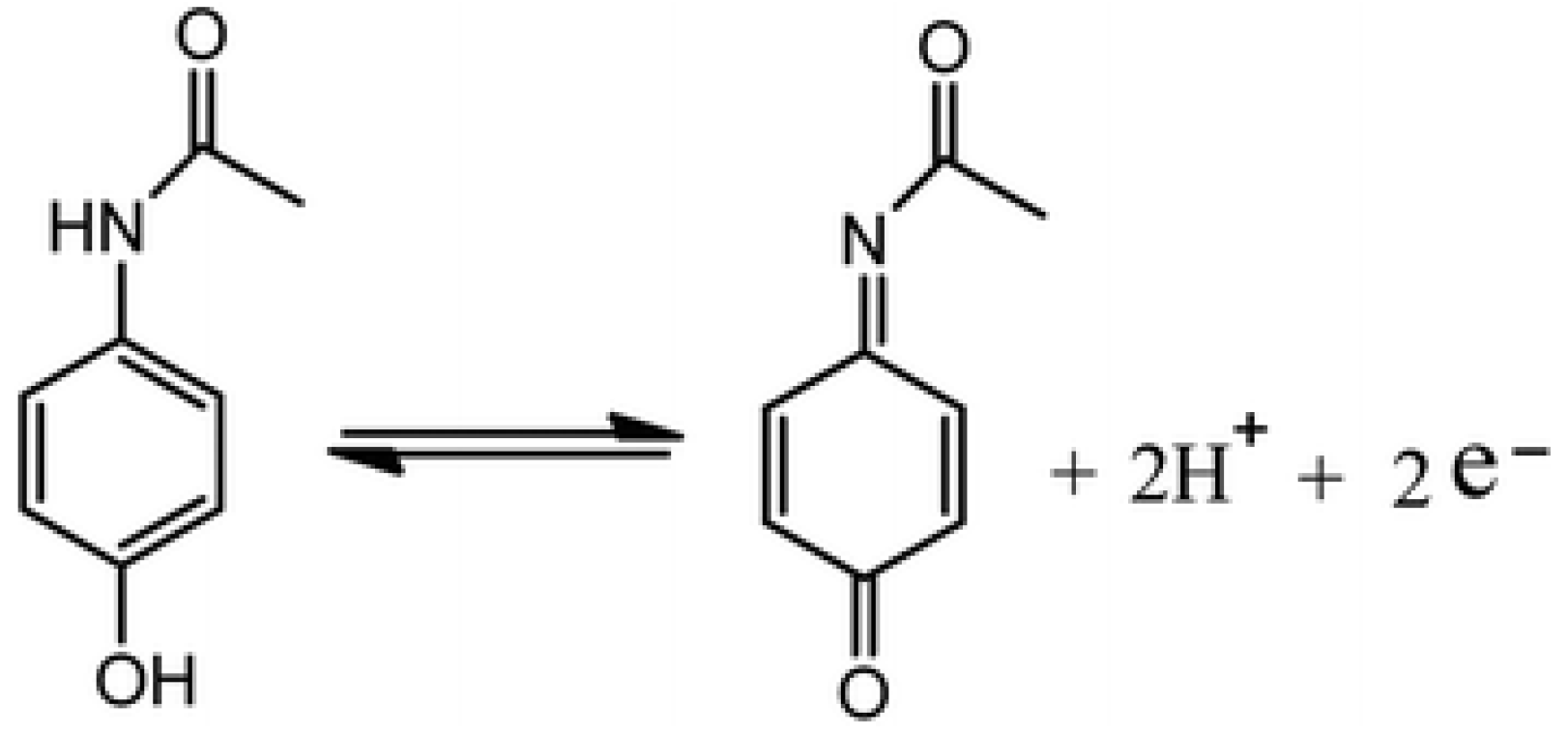
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Procedure
3. Results and Discussion
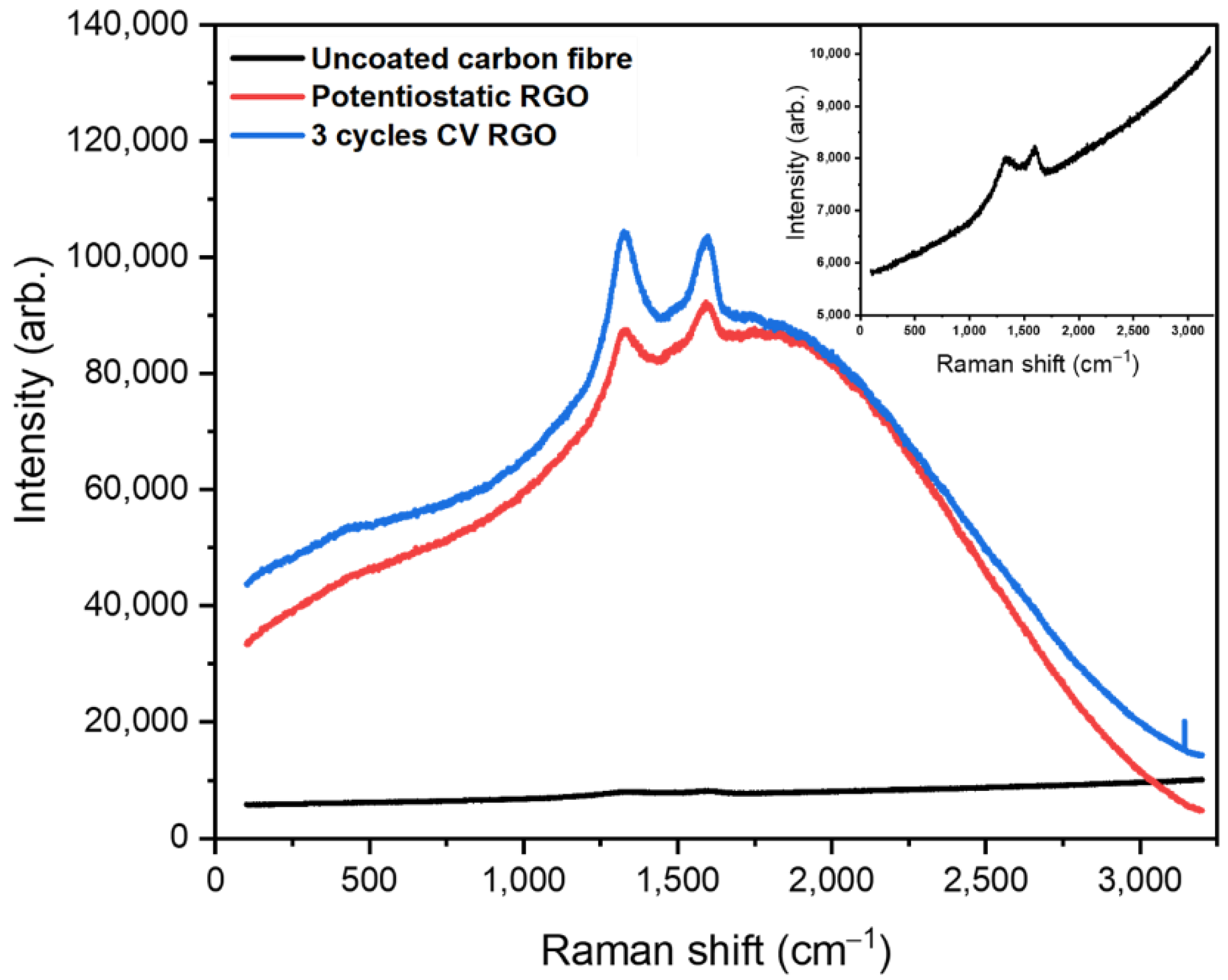
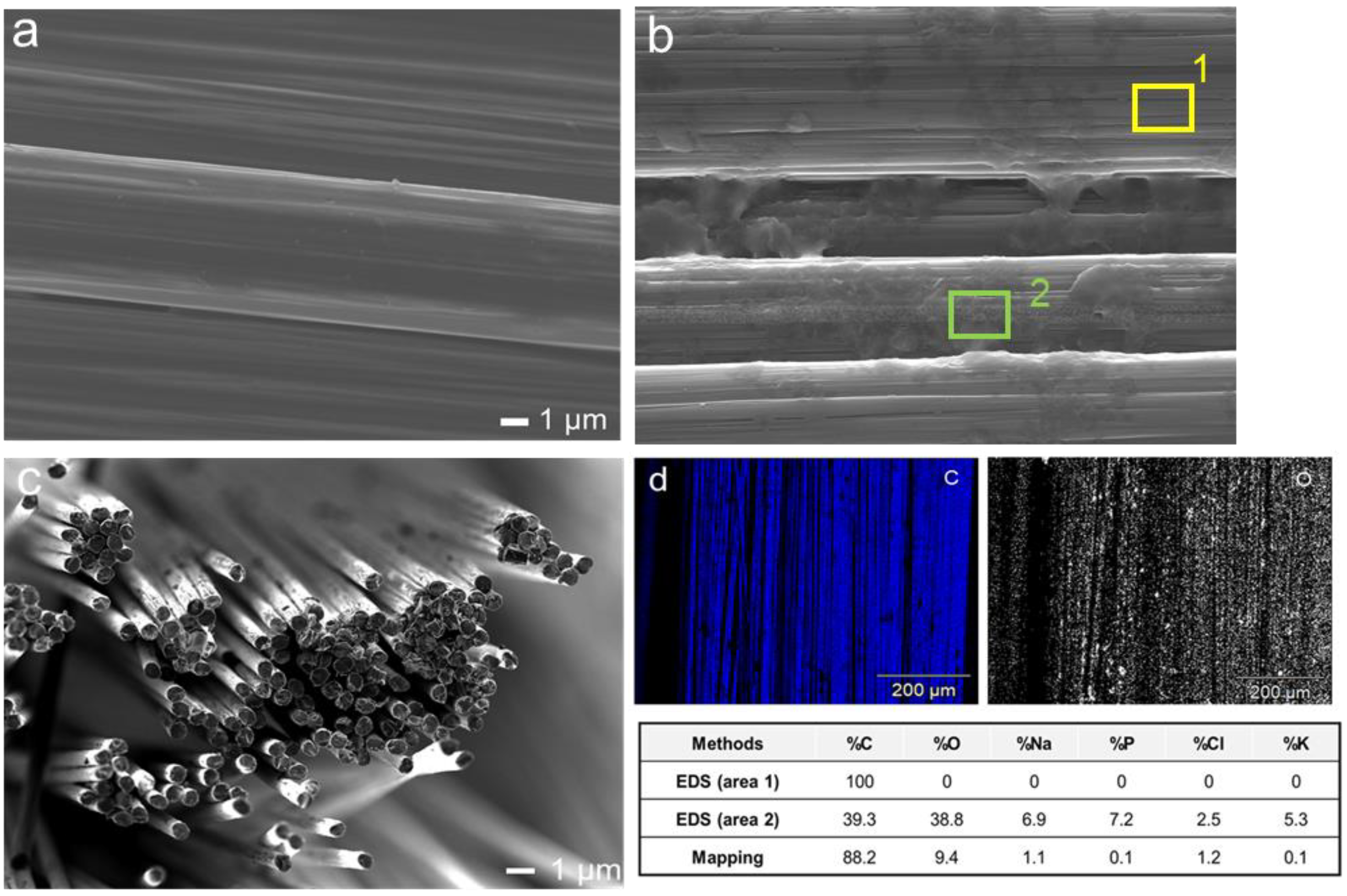
3.1. Characterisation
3.2. Surface Area of the Fibre Electrode
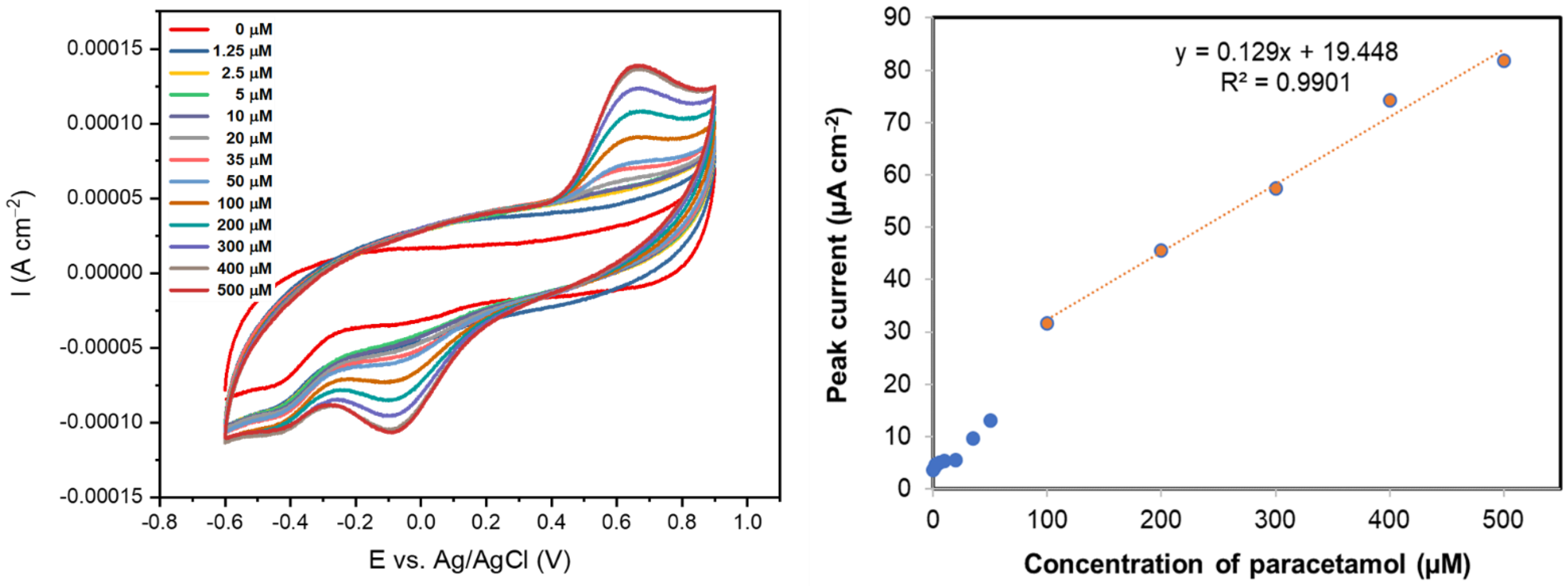
3.3. Drug Sensing
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Ghanbari, K.; Bonyadi, S. An electrochemical sensor based on reduced graphene oxide decorated with polypyrrole nanofibers and zinc oxide-copper oxide p-n junction heterostructures for the simultaneous voltammetric determination of ascorbic acid, dopamine, paracetamol, and tryptoph. New J. Chem. 2018, 42, 8512–8523. [Google Scholar] [CrossRef]
- Yang, W.; Chen, Y.; Wang, J.; Peng, T.; Xu, J.; Yang, B.; Tang, K. Reduced Graphene Oxide/Carbon Nanotube Composites as Electrochemical Energy Storage Electrode Applications. Nanoscale Res Lett. 2018, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Shen, C.; Lu, G.; Ni, Y.; Lu, C.; Xu, Z. Synthesis of PPy/RGO-based hierarchical material with super-paramagnetic behavior and understanding its robust photo current driven by visible light. Synth Met. 2018, 241, 17–25. [Google Scholar] [CrossRef]
- Ponnaiah, S.K.; Prakash, P.; Vellaichamy, B. A new analytical device incorporating a nitrogen doped lanthanum metal oxide with reduced graphene oxide sheets for paracetamol sensing. Ultrason Sonochem. 2018, 44, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Gu, S.; Ding, Y.; Jiang, F.; Zhang, Z. Facile and novel electrochemical preparation of a graphene–transition metal oxide nanocomposite for ultrasensitive electrochemical sensing of acetaminophen and phenacetin. Nanoscale 2014, 6, 207–214. [Google Scholar] [CrossRef] [PubMed]
| Uncoated Carbon Fibre | Potentiostatic | CV 3 | CV 5 | |
|---|---|---|---|---|
| Area of electrode (cm2) | 0.09 | 0.11 | 0.19 | 0.20 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sriprasertsuk, S.; Varcoe, J.R.; Crean, C. Reduced Graphene Oxide Fibre Electrodes for Drug Sensing. Proceedings 2021, 68, 18. https://doi.org/10.3390/proceedings2021068018
Sriprasertsuk S, Varcoe JR, Crean C. Reduced Graphene Oxide Fibre Electrodes for Drug Sensing. Proceedings. 2021; 68(1):18. https://doi.org/10.3390/proceedings2021068018
Chicago/Turabian StyleSriprasertsuk, Sutthima, John R. Varcoe, and Carol Crean. 2021. "Reduced Graphene Oxide Fibre Electrodes for Drug Sensing" Proceedings 68, no. 1: 18. https://doi.org/10.3390/proceedings2021068018
APA StyleSriprasertsuk, S., Varcoe, J. R., & Crean, C. (2021). Reduced Graphene Oxide Fibre Electrodes for Drug Sensing. Proceedings, 68(1), 18. https://doi.org/10.3390/proceedings2021068018





