Textile-Based Battery Using a Biodegradable Gel-Electrolyte †
Abstract
:1. Introduction
2. Experimental Section
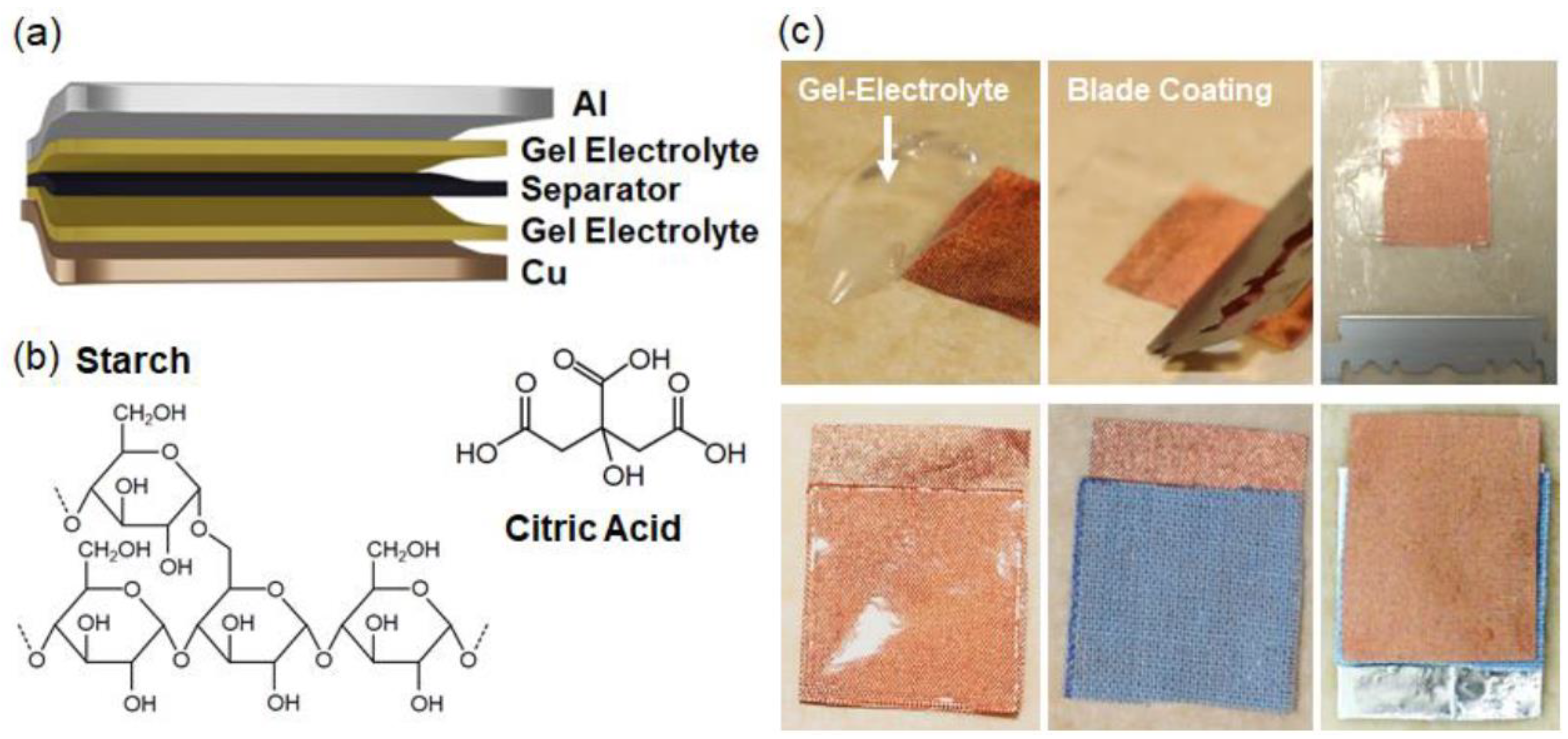
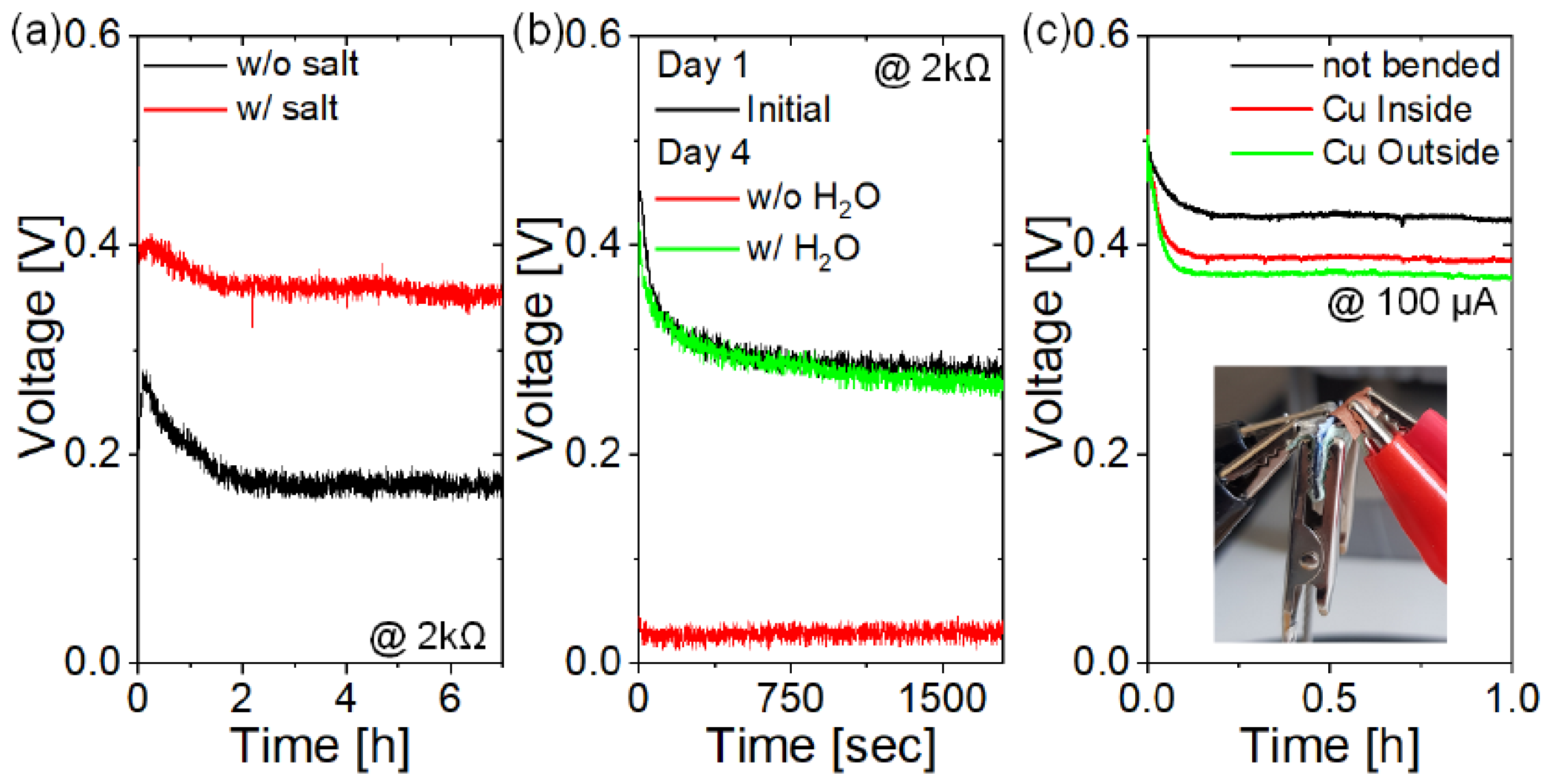
3. Results and Discussion
4. Conclusions
Supplementary Materials
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weng, W.; Chen, P.; He, S.; Sun, X.; Peng, H. Smart Electronic Textiles. Angew. Chem. Int. Ed. 2016, 55, 6140–6169. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, D.; Zheng, Z. Textile-Based Electrochemical Energy Storage Devices. Adv. Energy Mater. 2016, 6, 1600783. [Google Scholar] [CrossRef]
- Jost, K.; Dion, G.; Gogotsi, Y. Textile energy storage in perspective. J. Mater. Chem. A 2014, 2, 10776. [Google Scholar] [CrossRef]
- Chen, X.; Miao, L.; Guo, H.; Chen, H.; Song, Y.; Su, Z.; Zhang, H. Waterproof and stretchable triboelectric nanogenerator for biomechanical energy harvesting and self-powered sensing. Appl. Phys. Lett. 2018, 112, 203902. [Google Scholar] [CrossRef]
- Zhang, M.; Zhao, M.; Jian, M.; Wang, C.; Yu, A.; Yin, Z.; Liang, X.; Wang, H.; Xia, K.; Liang, X.; et al. Printable Smart Pattern for Multifunctional Energy-Management E-Textile. Matter 2019, 1, 168–179. [Google Scholar] [CrossRef]
- Kim, M.-K.; Kim, M.-S.; Lee, S.; Kim, C.; Kim, Y.-J. Wearable thermoelectric generator for harvesting human body heat energy. Smart Mater. Struct. 2014, 23, 105002. [Google Scholar] [CrossRef]
- Cho, S.H.; Lee, J.; Lee, M.J.; Kim, H.J.; Lee, S.-M.; Choi, K.C. Plasmonically Engineered Textile Polymer Solar Cells for High-Performance, Wearable Photovoltaics. ACS Appl. Mater. Interfaces 2019, 11, 20864–20872. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Z.; Liang, G.; Huang, Y.; Huang, Y.; Zhu, M.; Pei, Z.; Xue, Q.; Tang, Z.; Wang, Y.; et al. Waterproof and Tailorable Elastic Rechargeable Yarn Zinc Ion Batteries by a Cross-Linked Polyacrylamide Electrolyte. ACS Nano 2018, 12, 3140–3148. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Zhao, Y.; Hu, C.; Cheng, H.; Hu, Y.; Zhang, Z.; Shi, G.; Qu, L. All-Graphene Core-Sheath Microfibers for All-Solid-State, Stretchable Fibriform Supercapacitors and Wearable Electronic Textiles. Adv. Mater. 2013, 25, 2326–2331. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zeng, Y.; Zhang, H.; Yu, M.; Tong, Y.; Lu, X. Flexible Zn-Ion Batteries: Recent Progresses and Challenges. Small 2019, 15, 1804760. [Google Scholar] [CrossRef] [PubMed]
- Walter, M.; Kovalenko, M.V.; Kravchyk, K.V. Challenges and benefits of post-lithium-ion batteries. New J. Chem. 2020, 44, 1677–1683. [Google Scholar] [CrossRef]
- Zhu, Y.; Yuan, S.; Bao, D.; Yin, Y.; Zhong, H.; Zhang, X.; Yan, J.; Jiang, Q. Decorating Waste Cloth via Industrial Wastewater for Tube-Type Flexible and Wearable Sodium-Ion Batteries. Adv. Mater. 2017, 29, 1603719. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, B.; Zhong, C.; Liu, Z.; Liu, J.; Ma, L.; Deng, Y.; Han, X.; Wu, T.; Hu, W.; et al. Ultrathin Co 3 O 4 Layers with Large Contact Area on Carbon Fibers as High-Performance Electrode for Flexible Zinc-Air Battery Integrated with Flexible Display. Adv. Energy Mater. 2017, 7, 1700779. [Google Scholar] [CrossRef]
- Xu, Y.; Zhao, Y.; Ren, J.; Zhang, Y.; Peng, H. An All-Solid-State Fiber-Shaped Aluminum-Air Battery with Flexibility, Stretchability, and High Electrochemical Performance. Angew. Chem. Int. Ed. 2016, 55, 7979–7982. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gellner, S.; Schwarz-Pfeiffer, A.; Nannen, E. Textile-Based Battery Using a Biodegradable Gel-Electrolyte. Proceedings 2021, 68, 17. https://doi.org/10.3390/proceedings2021068017
Gellner S, Schwarz-Pfeiffer A, Nannen E. Textile-Based Battery Using a Biodegradable Gel-Electrolyte. Proceedings. 2021; 68(1):17. https://doi.org/10.3390/proceedings2021068017
Chicago/Turabian StyleGellner, Sandra, Anne Schwarz-Pfeiffer, and Ekaterina Nannen. 2021. "Textile-Based Battery Using a Biodegradable Gel-Electrolyte" Proceedings 68, no. 1: 17. https://doi.org/10.3390/proceedings2021068017
APA StyleGellner, S., Schwarz-Pfeiffer, A., & Nannen, E. (2021). Textile-Based Battery Using a Biodegradable Gel-Electrolyte. Proceedings, 68(1), 17. https://doi.org/10.3390/proceedings2021068017





