Novel Biodegradable Polyanhydrides Based on Betulin Disuccinate and Sebacic Acid for Medical Purpose †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Prepolymer and Polymer Synthesis
2.3. Characterization of Polyanhydrides
2.4. Hydrolytic Degradation of Copolymers
2.5. Cytostatic Activity of Polyanhydrides
2.6. Preparation and Characterization of Microspheres
3. Results and Discussion
3.1. Synthesis and Characterization of Betulin-Based Polyanhydrides
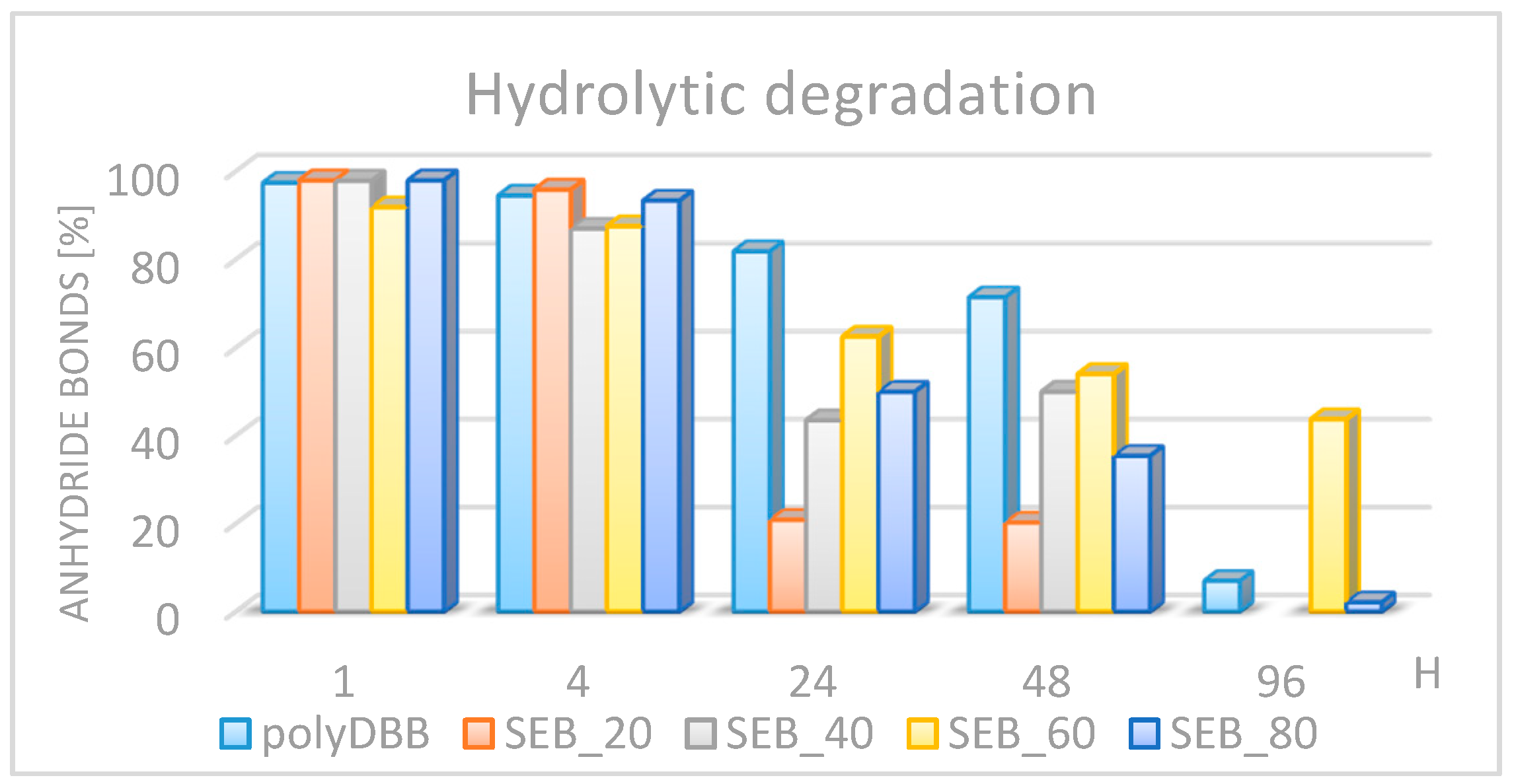
3.2. Hydrolytic Degradation of Polymers
3.3. Cytostatic Activity of Polyanhydrides
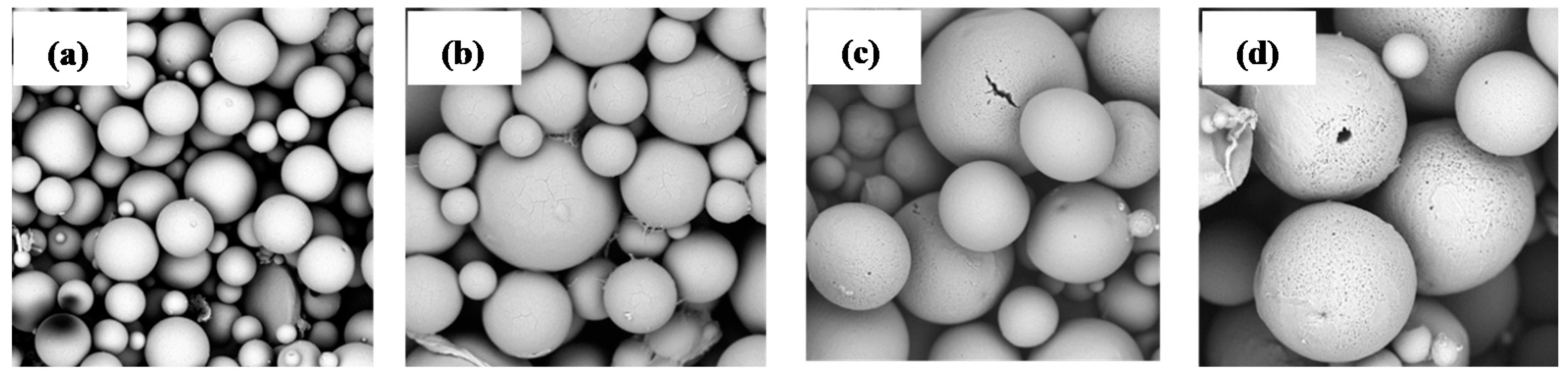
3.4. Microspheres Preparation and Characterization
Author Contributions
Funding
Conflicts of Interest
References
- Jeromenok, J.; Böhlmann, W.; Antonietti, M.; Weber, J. Intrinsically Microporous Polyesters From Betulin-Toward Renewable Materials for Gas Separation Made From Birch Bark. Macromol. Rapid Commun. 2011, 32, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Alakurtti, S.; Mäkelä, T.; Koskimies, S.; Yli-Kauhaluoma, J. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci. 2006, 29, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jeromenok, J.; Böhlmann, W.; Jäger, C.; Weber, J. Carbon Dioxide Adsorption in Betulin-Based Micro- and Macroporous Polyurethanes. ChemistryOpen 2013, 2, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Schlaad, H.; Weidner, S.; Antonietti, M. Synthesis of terpene–poly(ethylene oxide)s by t-BuP4-promoted anionic ring-opening polymerization. Polym. Chem. 2012, 3, 1763–1768. [Google Scholar] [CrossRef]
- Niewolik, D.; Krukiewicz, K.; Bednarczyk-Cwynar, B.; Ruszkowski, P.; Jaszcz, K. Novel polymeric derivatives of betulin with anticancer activity. RSC Adv. 2019, 9, 20892–20900. [Google Scholar] [CrossRef] [PubMed]
- Domb, A.J.; Kumar, N.; Ezra, A. Biodegradable Polymers in Clinical Use and Clinical Development; Wiley & Sons Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
| Compound | Cytostatic Activity IC50 [µg/mL] | |||||
|---|---|---|---|---|---|---|
| HeLa | A-549 | U-87 MG | KB | HepG2 | HDF | |
| DBB | 8.25 ± 0.81 | 7.09 ± 0.01 | 7.37 ± 0.26 | 7.17 ± 0.93 | 8.02 ± 0.04 | 14.80 ± 0.06 |
| polyDBB | 16.23 ± 0.72 | 16.19 ± 0.31 | 16.07 ± 0.02 | 17.81 ± 0.03 | 15.93 ± 0.12 | 27.13 ± 0.01 |
| SEB_20 | 4.61 ± 0.03 | 4.15 ± 0.24 | 4.99 ± 0.05 | 4.28 ± 0.01 | 4.15 ± 0.01 | 7.31 ± 1.92 |
| SEB_40 | 8.22 ± 0.09 | 8.15 ± 0.01 | 8.12 ± 0.91 | 8.04 ± 0.03 | 8.77 ± 0.23 | 13.55 ± 0.22 |
| SEB_60 | 8.10 ± 0.74 | 8.29 ± 0.55 | 8.41 ± 0.01 | 8.28 ± 0.01 | 8.39 ± 0.16 | 8,22 ± 1.17 |
| SEB_80 | 11.48 ± 0.22 | 11.29 ± 0.18 | 11.06 ± 0.37 | 11.39 ± 0.51 | 11.12 ± 0.46 | 18.01 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niewolik, D.; Bednarczyk-Cwynar, B.; Ruszkowski, P.; Jaszcz, K. Novel Biodegradable Polyanhydrides Based on Betulin Disuccinate and Sebacic Acid for Medical Purpose. Proceedings 2020, 67, 17. https://doi.org/10.3390/ASEC2020-07558
Niewolik D, Bednarczyk-Cwynar B, Ruszkowski P, Jaszcz K. Novel Biodegradable Polyanhydrides Based on Betulin Disuccinate and Sebacic Acid for Medical Purpose. Proceedings. 2020; 67(1):17. https://doi.org/10.3390/ASEC2020-07558
Chicago/Turabian StyleNiewolik, Daria, Barbara Bednarczyk-Cwynar, Piotr Ruszkowski, and Katarzyna Jaszcz. 2020. "Novel Biodegradable Polyanhydrides Based on Betulin Disuccinate and Sebacic Acid for Medical Purpose" Proceedings 67, no. 1: 17. https://doi.org/10.3390/ASEC2020-07558
APA StyleNiewolik, D., Bednarczyk-Cwynar, B., Ruszkowski, P., & Jaszcz, K. (2020). Novel Biodegradable Polyanhydrides Based on Betulin Disuccinate and Sebacic Acid for Medical Purpose. Proceedings, 67(1), 17. https://doi.org/10.3390/ASEC2020-07558







