Decomposition of Bromocresol Green Using a Nonthermal Atmospheric Pressure Plasma Jet †
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparing a Bromocresol Green Solution
2.2. Plasma Jet
2.3. Conductivity, pH, and Absorbance Measurement
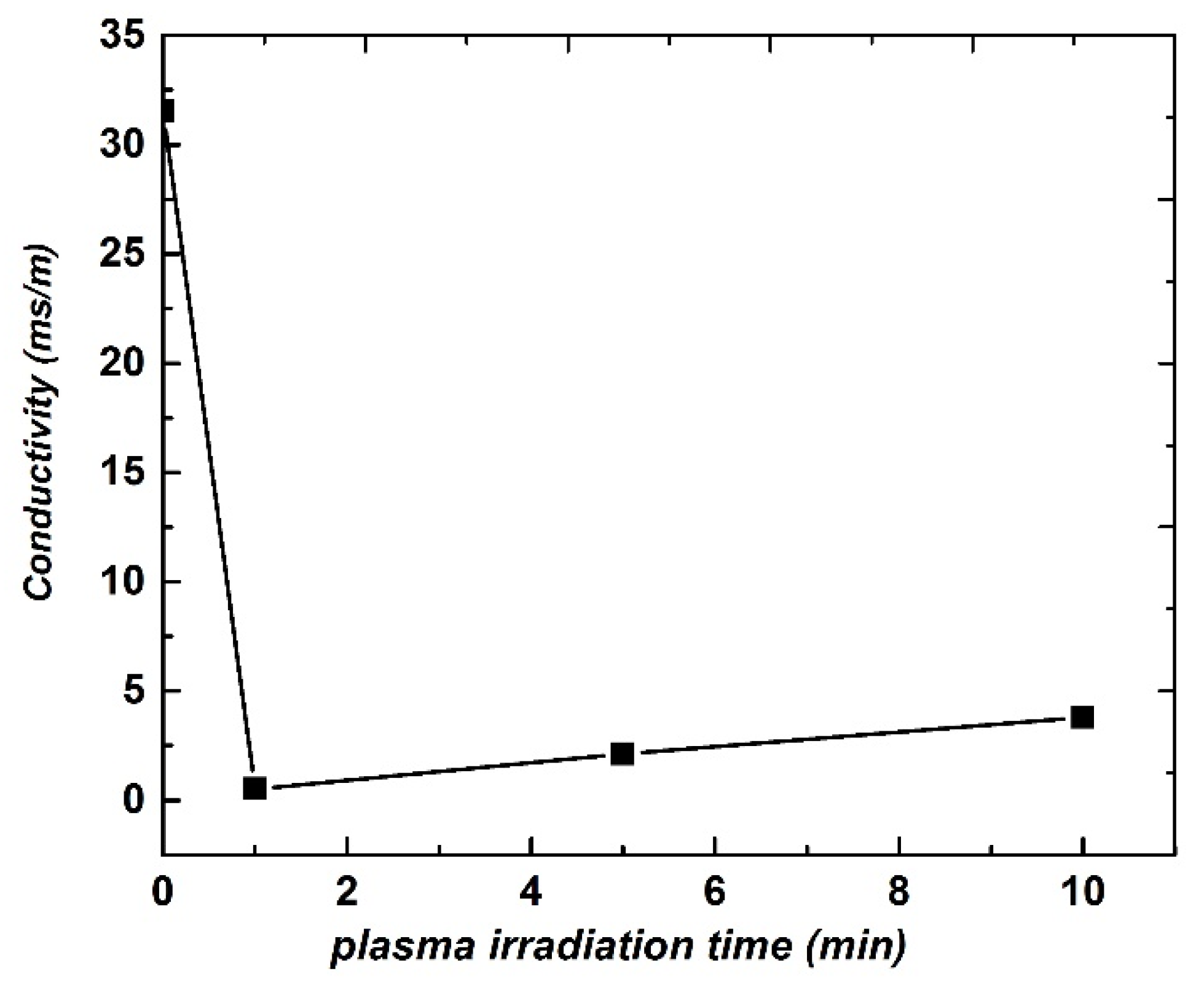
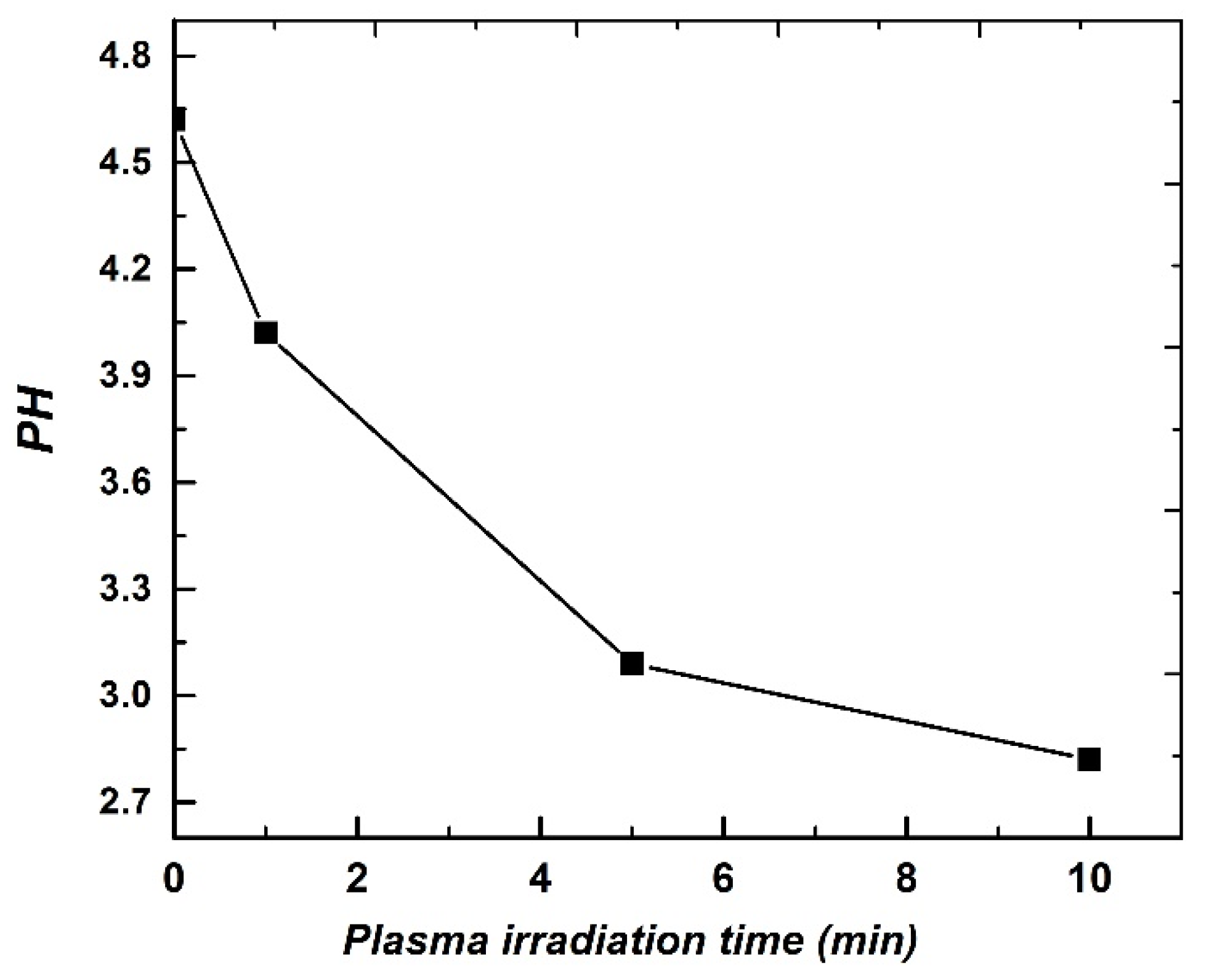
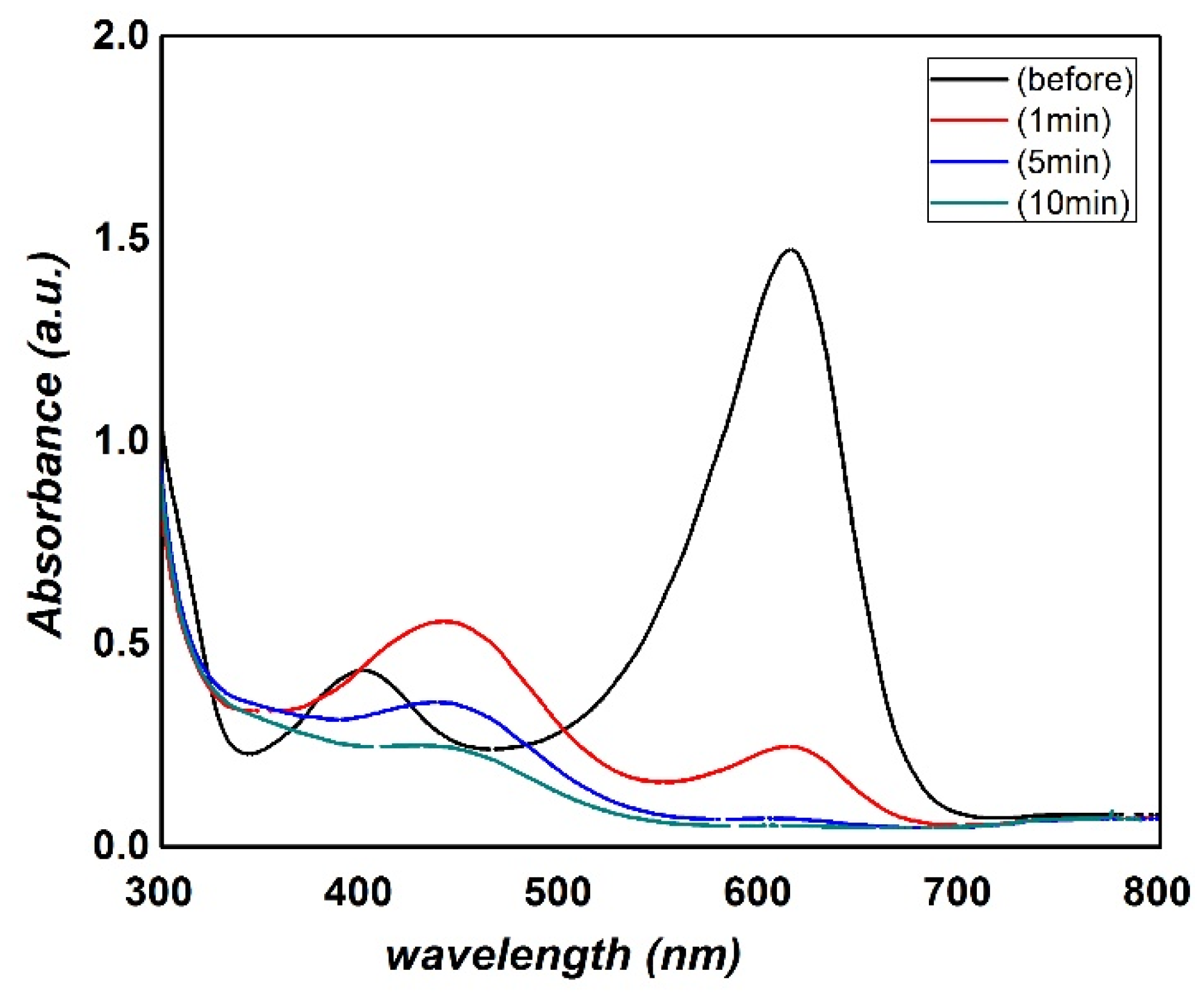
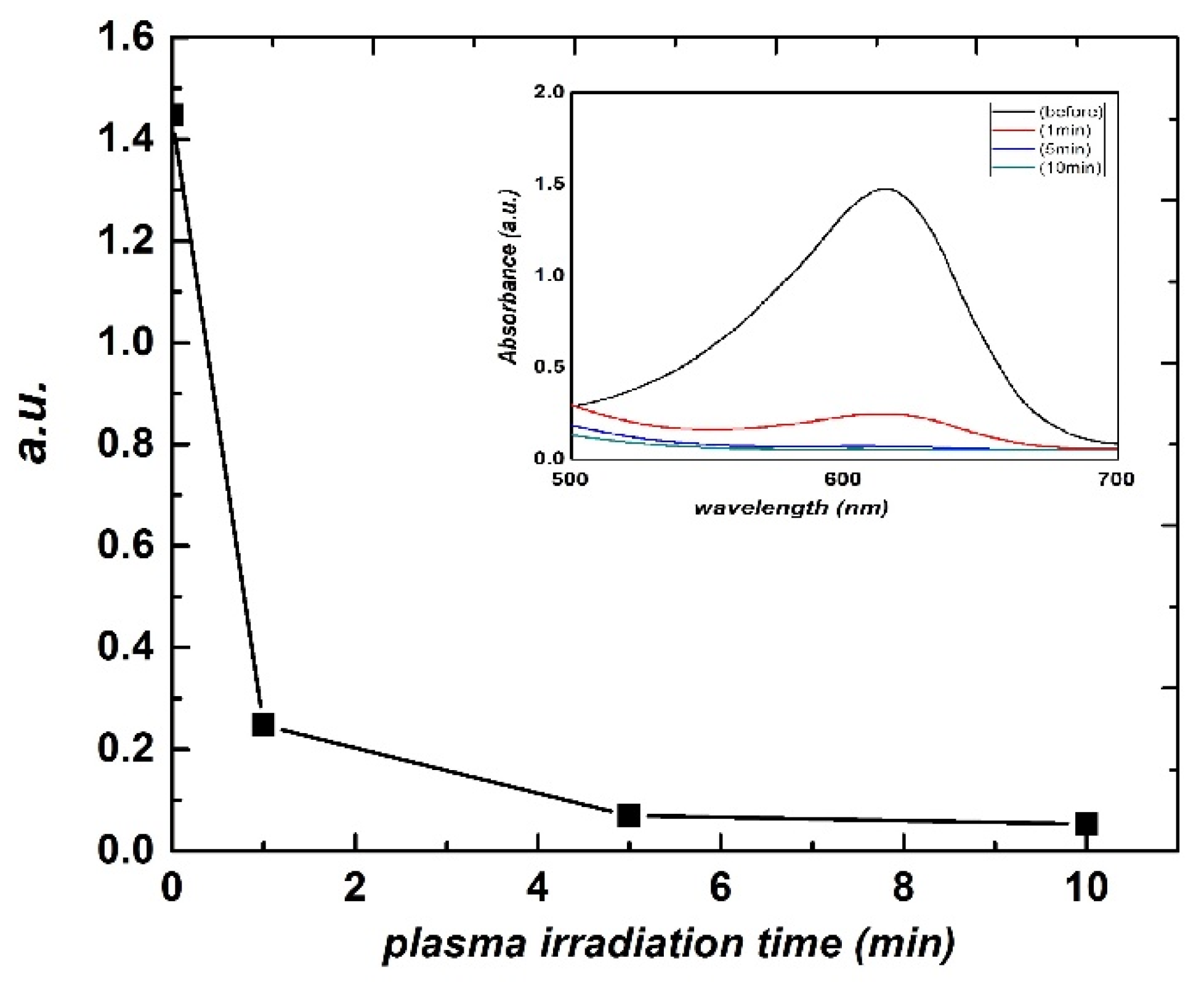
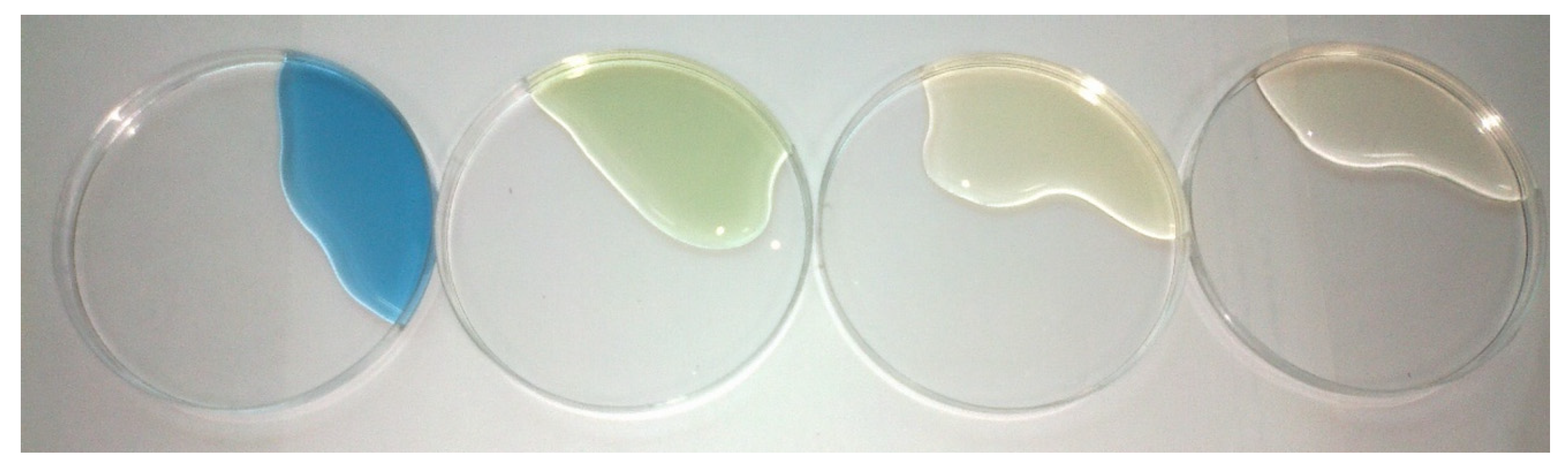
3. Results and Discussion
4. Conclusions
Funding
Conflicts of Interest
References
- Navaneetha Pandiyaraj, K.; Vasu, D.; Padmanabhan, P.V.A.; Pichumani, M.; Deshmukh, R.R.; Kandavelu, V. Evaluation of influence of cold atmospheric pressure argon plasma operating parameters on degradation of aqueous solution of Reactive Blue 198 (RB-198). Plasma Sci. Technol. 2020, 22, 055504. [Google Scholar] [CrossRef]
- Vasu, D.; Ramkumar, M.; Arunkumar, A. Cold Atmospheric Pressure Argon Plasma Jet Assisted Degradation of Malachite Green (MG) Aqueous Solution. Front. Adv. Mater. Res. 2020, 2, 51–61. [Google Scholar]
- Pandiyaraj, K.N.; Vasu, D.; Ghobeira, R.; Tabaei, P.S.E.; De Geyter, N.; Morent, R.; Pichumani, M.; Padmanabhanan, P.V.A.; Deshmukh, R.R. Dye wastewater degradation by the synergetic effect of an atmospheric pressure plasma treatment and the photocatalytic activity of plasma-functionalized Cu-TiO2 nanoparticles. J. Hazard. Mater. 2020, 124264. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-M.; Patange, A.; Sun, D.-W.; Tiwari, B. Plasma-activated water: Physicochemical properties, microbial inactivation mechanisms, factors influencing antimicrobial effectiveness, and applications in the food industry. Compr. Rev. Food Sci. Food Saf. 2020. [Google Scholar] [CrossRef] [PubMed]
- Al-rawaf, A.F.; Fuliful, F.K.; Khalaf, M.K.; Oudah, H.K. Studying the non-thermal plasma jet characteristics and application on bacterial decontamination. J. Theor. Appl. Phys. 2018, 12, 45–51. [Google Scholar] [CrossRef]
- Abbasi, H.; Nazeri, M.; Mirpour, S.; Farahani, N.J. Measuring electron density, electric field and temperature of a micro-discharge air plasma jet using optical emission spectroscopy. In Proceedings of the 2015 2nd International Conference on Knowledge-Based Engineering and Innovation (KBEI), Tehran, Iran, 5–6 November 2015; pp. 1139–1141. [Google Scholar]
- Hayashi, Y.; Wahyudiono, *!!! REPLACE !!!*; Machmudah, S.; Takada, N.; Kanda, H.; Sasaki, K.; Goto, M. Decomposition of methyl orange using pulsed discharge plasma at atmospheric pressure: Effect of different electrodes. Jpn. J. Appl. Phys. 2013, 53, 010212. [Google Scholar] [CrossRef]
- Magureanu, M.; Piroi, D.; Gherendi, F.; Mandache, N.B.; Parvulescu, V. Decomposition of methylene blue in water by corona discharges. Plasma Chem. Plasma Process. 2008, 28, 677–688. [Google Scholar] [CrossRef]
- Krugly, E.; Martuzevicius, D.; Tichonovas, M.; Jankunaite, D.; Rumskaite, I.; Sedlina, J.; Racys, V.; Baltrusaitis, J. Decomposition of 2-naphthol in water using a non-thermal plasma reactor. Chem. Eng. J. 2015, 260, 188–198. [Google Scholar] [CrossRef]
- Matinzadeh, Z.; Shahgoli, F.; Abbasi, H.; Salem, M.K.; Ghoranneviss, M. Effect of the Non-Thermal Argon Plasma Irradiation on Decolorization of Methyl Orange Solution. In Proceedings of the Second Iranian Conference on Engineering Electromagnetics (ICEEM 2014), Tehran Iran, 8–9 January 2014; pp. 1–6. [Google Scholar]
- Matinzadeh, Z.; Shahgoli, F.; Abbasi, H.; Ghoranneviss, M.; Salem, M.K. The Effect of Argon/Nitrogen Plasma Jet Irradiation on Optical Properties of Distilled Water. Int. J. Mechatron. Electr. Comput. Technol. (IJMEC) 2018, 8, 6. [Google Scholar]
- Matinzadeh, Z.; Shahgoli, F.; Abbasi, H.; Ghoranneviss, M.; Salem, M.K. Degradation of bromophenol blue molecule during argon plasma jet irradiation. J. Theor. Appl. Phys. 2017, 11, 97–102. [Google Scholar] [CrossRef]
- Zarei, T.; Mirpour, S.; Nikmaram, H.; Ghoranneviss, M.; Mirpour, S.; Dorranian, D. Softening Hard Water Using High Frequency Spark Plasma Discharge. Plasma Chem. Plasma Process. 2017, 37, 99–114. [Google Scholar] [CrossRef]
- Zarei, T.; Dorranian, D. Investigating the Optimized Physical and Electrical Operating Condition of DC Pulsed Spark Discharge Over Water Surface Generated by Different Input Parameters. IEEE Trans. Plasma Sci. 2019, 47, 3949–3959. [Google Scholar] [CrossRef]
- Gharagozalian, M.; Dorranian, D.; Ghoranneviss, M. Water treatment by the AC gliding arc air plasma. J. Theor. Appl. Phys. 2017, 11, 171–180. [Google Scholar] [CrossRef]
- Nezamzadeh-Ejhieh, A.; Moazzeni, N. Sunlight photodecolorization of a mixture of Methyl Orange and Bromocresol Green by CuS incorporated in a clinoptilolite zeolite as a heterogeneous catalyst. J. Ind. Eng. Chem. 2013, 19, 1433–1442. [Google Scholar] [CrossRef]
- Fassi, S.; Djebbar, K.; Sehili, T. Photocatalytic Degegradation of Bromocresol green by TiO2/UV in aqueous medium. J. Mater. Environ. Sci. 2014, 5, 1093–1098. [Google Scholar]
- Lu, Y.; Wei, B.; Wang, Y.; Li, J. Studies on the Removal of Bromocresol Green from Water by Solvent Sublation. Sep. Sci. Technol. 2007, 42, 1901–1911. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Alizadeh, A.; Malekhosseini, Z.; Ranjbar, M. Removal of Bromocresol Green from Aqueous Solution via Adsorption on Ziziphus nummularia as a New, Natural, and Low-Cost Adsorbent: Kinetic and Thermodynamic Study of Removal Process. J. Chem. Eng. Data 2011, 56, 3738–3746. [Google Scholar] [CrossRef]
- Ghaedi, M.; Khajesharifi, H.; Hemmati Yadkuri, A.; Roosta, M.; Sahraei, R.; Daneshfar, A. Cadmium hydroxide nanowire loaded on activated carbon as efficient adsorbent for removal of Bromocresol Green. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 86, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kuwahata, H.; Kimura, K.; Ohyama, R.-I. Decolorization of Methylene Blue Aqueous Solution by Atmospheric-Pressure Plasma Jet. e-J. Surf. Sci. Nanotechnol. 2010, 8, 381–383. [Google Scholar] [CrossRef][Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matinzadeh, Z.; Shahgoli, F.; Abbasi, H.; Ghoranneviss, M.; Salem, M.K. Decomposition of Bromocresol Green Using a Nonthermal Atmospheric Pressure Plasma Jet. Proceedings 2020, 67, 14. https://doi.org/10.3390/ASEC2020-07513
Matinzadeh Z, Shahgoli F, Abbasi H, Ghoranneviss M, Salem MK. Decomposition of Bromocresol Green Using a Nonthermal Atmospheric Pressure Plasma Jet. Proceedings. 2020; 67(1):14. https://doi.org/10.3390/ASEC2020-07513
Chicago/Turabian StyleMatinzadeh, Ziba, Farhad Shahgoli, Hamed Abbasi, Mahmood Ghoranneviss, and Mohammad K. Salem. 2020. "Decomposition of Bromocresol Green Using a Nonthermal Atmospheric Pressure Plasma Jet" Proceedings 67, no. 1: 14. https://doi.org/10.3390/ASEC2020-07513
APA StyleMatinzadeh, Z., Shahgoli, F., Abbasi, H., Ghoranneviss, M., & Salem, M. K. (2020). Decomposition of Bromocresol Green Using a Nonthermal Atmospheric Pressure Plasma Jet. Proceedings, 67(1), 14. https://doi.org/10.3390/ASEC2020-07513




