Lactococcus lactis RBT18: From the Rainbow Trout Farm to the Lab, the Tale of a Nisin Z Producer †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Antimicrobial Activity
2.3. PCR-Amplification of the Bacteriocin Structural Gene
2.4. Cross Immunity Assays
2.5. Bacteriocin Purification Procedures
Reversed Phase-Fast Protein Liquid Chromatography (RP-FPLC)
2.6. Mass Spectrometry Analysis (MALDI-TOF/TOF)
3. Results and Discussion
3.1. Antimicrobial Activity
3.2. PCR-Amplification of the Extracellular Antimicrobial Compound (Bacteriocin) Structural Gene
3.3. Cross Immunity Assay
3.4. RP-FPLC Bacteriocin Purification and MALDI-TOF/TOF Mass Spectrometry Analyses
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; FAO Fisheries and Aquaculture Department: Rome, Italy, 2020. [Google Scholar]
- Nayak, S.K. Probiotics and immunity: A fish perspective. Fish Shellfish Immunol. 2010, 29, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sánchez, T.; Ruiz-Zarzuela, I.; de Blas, I.; Balcázar, J.L. Probiotics in aquaculture: A current assessment. Rev. Aquac. 2014, 5, 1–14. [Google Scholar]
- Vendrell, D.; Balcázar, J.L.; Ruiz-Zarzuela, I.; de Blas, I.; Gironés, O.; Múrquiz, J.L. Lactococcus garvieae in fish: A review. Comp. Immunol. Microbiol. Infect. Dis. 2006, 29, 177–198. [Google Scholar] [CrossRef] [PubMed]
- Cintas, L.M.; Casaus, M.P.; Herranz, C.; Nes, I.F.; Hernández, P.E. Review: Bacteriocins of lactic acid bacteria. Food Sci. Tech. Int. 2001, 7, 281–305. [Google Scholar] [CrossRef]
- Gómez-Sala, B.; Feito, J.; Hernández, P.E.; Cintas, L.M. Lactic acid bacteria in aquatic environments and their applications. In Lactic Acid Bacteria: Microbiological and Functional Aspects, 5th ed.; Vinderola, G., Ouwehand, A.C., Salminen, S., von Wright, A., Eds.; CRC Press: Boca Raton, FL, USA, 2019; pp. 555–578. [Google Scholar]
- Zendo, T.; Yoneyama, F.; Sonomoto, K. Lactococcal membrane-permeabilizing antimicrobial peptides. Appl. Microbiol. Biotechnol. 2010, 88, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.; Muñoz-Atienza, E.; Nahuelquín, Y.; Poeta, P.; Igrejas, G.; Hernández, P.E.; Herranz, C.; Cintas, L.M. Inhibition of fish pathogens by the microbiota from rainbow trout (Oncorhynchus mykiss, Walbaum) and rearing environment. Anaerobe 2015, 32, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Araújo, C.; Muñoz-Atienza, E.; Pérez-Sánchez, T.; Poeta, P.; Igrejas, G.; Hernández, P.E.; Herranz, C.; Ruiz-Zarzuela, I.; Cintas, L.M. Nisin Z production by Lactococcus lactis subsp. cremoris WA2-67 of aquatic origin as a defense mechanism to protect rainbow trout (Oncorhynchus mykiss, Walbaum) against Lactococcus garvieae. Mar. Biotechnol. 2015, 17, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Saavedra, L.; Castellano, P.; Sesma, F. Purification of bacteriocins produced by Lactic Acid Bacteria. Methods in Molecular Biology. In Public Health Microbiology: Methods and Protocols; Spencer, J.F.T., de Spencer, A.L.R., Eds.; Humana Press Inc.: Totowa, NJ, USA; Volume 268, pp. 331–336.
- Guyonet, D.; Fremaux, C.; Cenatiempo, Y.; Berjeaud, J.M. Method for rapid purification of class IIa bacteriocins and comparison of their activities. Appl. Environ. Microbiol. 2000, 66, 1744–1748. [Google Scholar] [CrossRef] [PubMed]
- Cintas, L.M. Caracterización Bioquímica y Genética Parcial de la Pediocina L50, Una Nueva Bacteriocina Producida Por Pediococcus Acidilactici L50 Aislado de Embutidos Crudos Curados. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 1995. [Google Scholar]
- Cintas, L.M.; Rodríguez, J.M.; Fernández, M.F.; Sletten, K.; Nes, I.F.; Hernández, P.E.; Holo, H. Isolation and characterization of pediocin L50, a new bacteriocin from Pediococcus acidilactici with a broad inhibitory spectrum. Appl. Environ. Microbiol. 1995, 61, 2643–2648. [Google Scholar] [CrossRef] [PubMed]
- Suda, S.; Field, D.; Barron, N. Antimicrobial Peptide Production and Purification. In Protein Chromatography. Methods in Molecular Biology; Walls, D., Loughran, S., Eds.; Humana Press: New York, NY, USA. [CrossRef]
- Wilson-Stanford, S.; Kalli, A.; Håkansson, K.; Kastrantas, J.; Orugunty, R.S.; Smith, L. Oxidation of lanthionines renders the lantibiotic nisin inactive. Appl. Environ. Microbiol. 2009, 75, 1381–1387. [Google Scholar] [CrossRef] [PubMed]

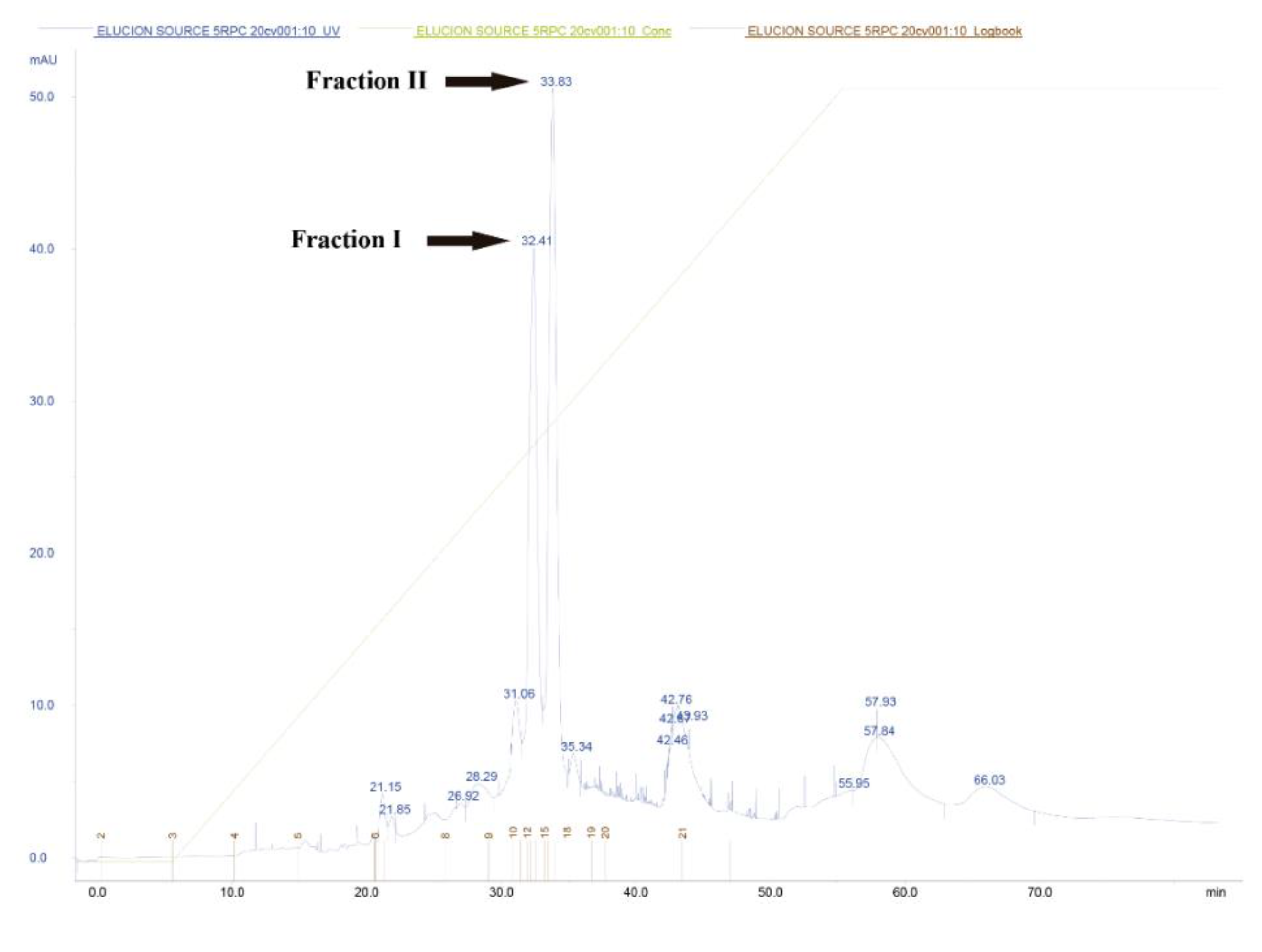
| Volume (ml) | Total A280a | Total Activity (103 BU b | Specific Activityc | Increase in Specific Activityd | Yield (%)e | |
|---|---|---|---|---|---|---|
| Procedure 1 | ||||||
| Fraction I | 1.5 | 0.031 | 983 | 31,700,000 | 348,000 | 77 |
| Procedure 2 | ||||||
| Fraction I | 0.5 | 0.010 | 10 | 975,000 | 2700 | 0.4 |
| Fraction II | 0.6 | 0.007 | 98 | 12,600,000 | 35,000 | 3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contente, D.; Feito, J.; Borrero, J.; Peña, N.; Muñoz-Atienza, E.; Igrejas, G.; Poeta, P.; Hernández, P.E.; Cintas, L.M. Lactococcus lactis RBT18: From the Rainbow Trout Farm to the Lab, the Tale of a Nisin Z Producer. Proceedings 2020, 66, 8. https://doi.org/10.3390/proceedings2020066008
Contente D, Feito J, Borrero J, Peña N, Muñoz-Atienza E, Igrejas G, Poeta P, Hernández PE, Cintas LM. Lactococcus lactis RBT18: From the Rainbow Trout Farm to the Lab, the Tale of a Nisin Z Producer. Proceedings. 2020; 66(1):8. https://doi.org/10.3390/proceedings2020066008
Chicago/Turabian StyleContente, Diogo, Javier Feito, Juan Borrero, Nuria Peña, Estefanía Muñoz-Atienza, Gilberto Igrejas, Patrícia Poeta, Pablo E. Hernández, and Luis M. Cintas. 2020. "Lactococcus lactis RBT18: From the Rainbow Trout Farm to the Lab, the Tale of a Nisin Z Producer" Proceedings 66, no. 1: 8. https://doi.org/10.3390/proceedings2020066008
APA StyleContente, D., Feito, J., Borrero, J., Peña, N., Muñoz-Atienza, E., Igrejas, G., Poeta, P., Hernández, P. E., & Cintas, L. M. (2020). Lactococcus lactis RBT18: From the Rainbow Trout Farm to the Lab, the Tale of a Nisin Z Producer. Proceedings, 66(1), 8. https://doi.org/10.3390/proceedings2020066008










