1. Introduction
Candida spp. are part of normal human microbiome but are important opportunistic pathogens. They have been reported to cause superficial and systemic infections in immunocompromised and susceptible individuals [
1].
The increasing incidences of drug resistance among pathogens is the major threat for the human health, which highlight the need for discovery of new therapeutic agents as potential drug candidates. Actinobacteria in general and members of the genus,
Streptomyces in particular have been well recognized for their ability to produce a plethora of antibiotics. It is well known that the
Streptomyces spp. contribute about 80% in the production of bioactive compounds out of all actinobacteria [
2]. The structural and functional diversity of secondary metabolites makes them an inexhaustible resource for the drug discovery [
3].
The production of secondary metabolites in the microorganisms is governed by biosynthetic gene clusters whose expression can be modulated by factors like carbon and nitrogen sources and pH [
4]. The composition of complex nitrogen sources like yeast extract and peptone sourced from different manufacturers might vary in respect of the composition of amino acids and peptides [
5]. Bacteriological peptone is an enzymatic digest of animal tissues (meat). It has a high content of tryptophan along with the presence of other amino acids that supports the growth of the microorganisms including
Streptomyces chrestomyceticus [
6]. However, the composition of peptone depends on the source and condition of extraction that may vary from manufacture to manufacture and even in among lots of the same manufacturer [
7].
In the present study, the effect of peptone, procured from different manufacturers, on the production antifungal metabolites by S. chrestomyceticus ADP4 with activities against C. albicans, C. krusei, C. tropicalis, C. parapsilosis and C. auris is reported.
2. Methods
S. chrestomyceticus strain ADP4, used to produce secondary metabolites, has been described earlier [
6]. Culture was maintained at 28 °C and stored at 4 °C on Nutrient Agar (pH 7.0). Test pathogens were
C. albicans ATCC 10231,
C. krusei ATCC 6258,
C. tropicalis ATCC 750,
C. parapsilosis ATCC 90028 and
C. auris CBS 12372, which were maintained at 37 °C on Sabouraud Dextrose Agar (SDA, pH 5.6).
Single colony of 48 h grown ADP4 culture was transferred to 3 mL SDB and incubated at 28 °C and 150 rpm for 72 h. This inoculum was transferred to 300 mL SDB for production of secondary metabolites. The SDB was prepared with peptone procured from different brands: Himedia (Mumbai, India), Diffco (New Jersey, USA) and CDH (New Delhi, India). Production was carried out for a period of 4 days under the conditions mentioned above. The metabolites were extracted, and their anti-
Candia activities were characterized as reported earlier [
6]. Metabolite profiling of the extract was done by using reverse phase HPLC on C18 column.
3. Results and Discussion
The maximum zone of inhibition was observed against all the test pathogens on day 4 in the culture broth of SDB with Himedia- peptone and Diffco- peptone. However, the highest activity was observed on day 4 that continues till day 8. Therefore, all the cultures were harvested on day 4. The interesting observation was recorded in SDB with CDH-peptone that activity increased drastically against all the test pathogens. However, the activity against C. krusei ATCC 6258 had seen remarkable increase. Furthermore, highest dry cell mass of 205 mg/L was obtained in SDB with CDH-peptone followed by 87.3 mg/L and 48.5 mg/L in SDB with the peptone from Diffco and Himedia, respectively.
The liquid–liquid extraction of all the broths were active against all the mentioned test pathogens. The yield of the metabolite extract was found to be highest in the SDB with CDH-peptone (323 mg/L) as compared to the SDB with Himedia-peptone (202 mg/L) and with Diffco-peptone (185 mg/L).
As the results from well diffusion assay were semi-qualitative, a more accurate estimation of the potency of the extracts was obtained by determining MIC
90 values, which are presented in
Table 1.
The data suggest that the SDB with CDH-peptone is highly potent having very low MIC
90 value as compared to the SDB with Himedia-peptone against all the test pathogens. Furthermore, the activity of SDB with CDH-peptone was found to be higher as compared to the recently reported
Streptomyces spp. [
7,
8].
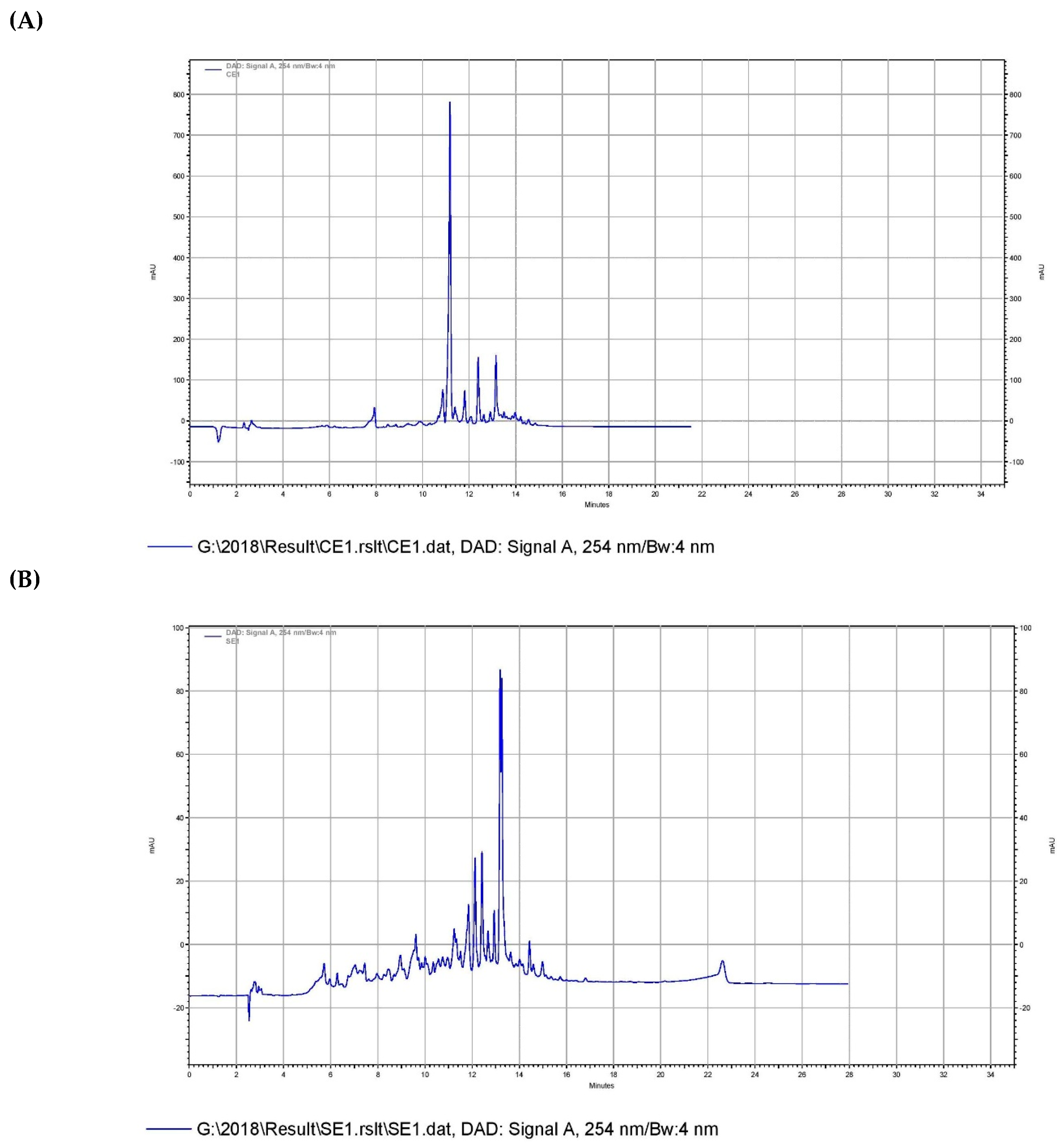
To look for the reason behind the significant difference in the potency of these extracts, the analytical RP-HPLC was performed for SDB with CDH-peptone and SDB with Himedia-peptone
. The chromatogram of RP-HPLC clearly indicates the difference among the metabolite profile of two extracts as shown in
Figure 1.
The results clearly indicated the difference in the metabolite profiles of the extracts that may confer difference in their potency. From overall results of the yield and activity profile, it can be suggested that the same component of culture medium like peptone but from different brands have significant effect on S. chretomyceticus strain ADP4 physiology, as well as in the production different of metabolites in the medium that vary in their potency. However, clearer reason behind the difference in the metabolite production by strain ADP4 is required to be investigated further.
4. Conclusions
Peptone procured from different brands had a remarkable effect on the production of several anti-fungal metabolites in S. chretomyceticus strain ADP4. Since peptone primarily served as complex source of nitrogen in the medium, it can be inferred that the expression of biosynthetic gene clusters (BGCs) in S. chrestomyceticus strain ADP4 can be modulated by nitrogen. However, the precise and predictable regulation of BGCs for production of specific or anti-Candida compounds need in-depth investigation.
Author Contributions
A.K.D. has conceptualized the research project and guided the experimental work. R.S. has carried out the experiments and prepared the initial draft of the manuscript, which was corrected and finalized by A.K.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by DST-SERB, India, DST-SERB EMR/2017/000254. No funding for APC was provided by any agency.
Acknowledgments
The research grant by the DST—SERB, Government of India to carry out this work is duly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Naglik, J.R.; König, A.; Hube, B.; Gaffen, S.L. Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr. Opin. Microbiol. 2017, 40, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Bérdy, J. Thoughts and facts about antibiotics: Where we are now and where we are heading. J. Antibiot. 2012, 65, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Procópio, R.E.; Silva, I.R.; Martins, M.K.; Azevedo, J.L.; Araújo, J.M. Antibiotics produced by Streptomyces. Braz. J. Infect. Dis. 2012, 16, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A. Regulation of fungal secondary metabolism. Nat. Rev. Microbiol. 2012, 11, 21–32. [Google Scholar] [CrossRef]
- Sørensen, K.; Van den Broucke, S.; Pelikan, J.M.; Fullam, J.; Doyle, G.; Slonska, Z.; Kondilis, B.; Stoffels, V.; Osborne, R.H.; Brand, H. Measuring health literacy in populations: Illuminating the design and development process of the European Health Literacy Survey Questionnaire (HLS-EU-Q). BMC Public Health. 2013, 13, 948. [Google Scholar] [CrossRef]
- Srivastava, V.; Dubey, A.K. Anti-biofilm activity of the metabolites of Streptomyces chrestomyceticus strain ADP4 against Candida albicans. J. Biosci. Bioeng. 2016, 122, 434–440. [Google Scholar] [CrossRef]
- Escalante-Réndiz, D.; de-la-Rosa-García, S.; Tapia-Tussell, R.; Martín, J.; Reyes, F.; Vicente, F.; Gamboa-Angulo, M. Molecular Identification of Selected Streptomyces Strains Isolated from Mexican Tropical Soils and their Anti-Candida Activity. Int. J. Environ. Res. Public. Health. 2019, 16, 1913. [Google Scholar] [CrossRef] [PubMed]
- Ambarwati, A.; Wahyuono, S.; Moeljopawiro, S.; Yuwono, T. Antimicrobial activity of ethyl acetate extracts of Streptomyces sp. CRB46 and the prediction of their bioactive compounds chemical structure. Biodiversitas 2019, 20. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).