Cultivation of Microalgae Chlorella Using Wine Industry by-Products †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Conditions
2.2. Analytical Determinations
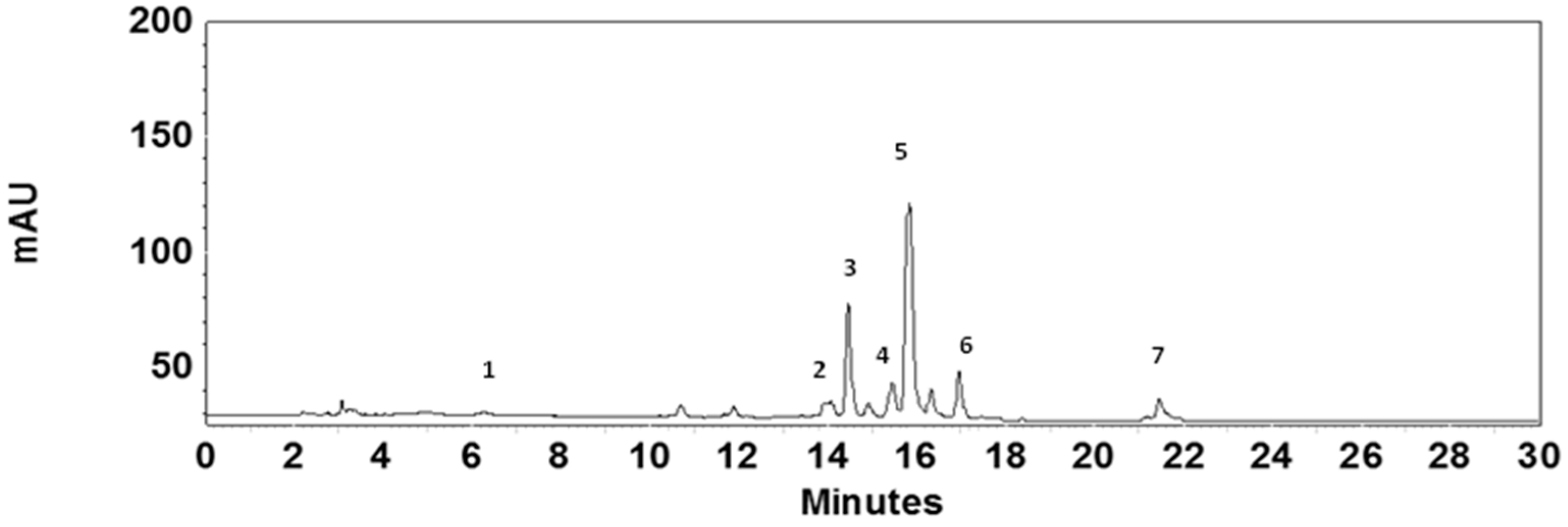
2.3. Pigment and Antioxidant Activity Analysis
2.4. Dry Weight Measurements
2.5. Maximum Photosystem II Quantum Yield
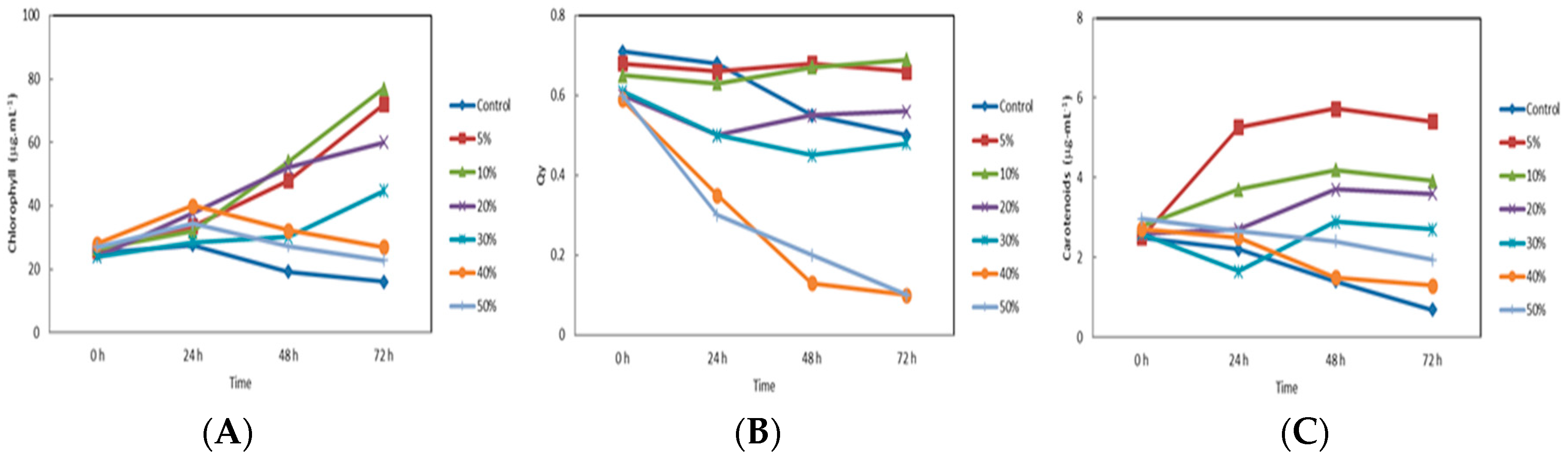
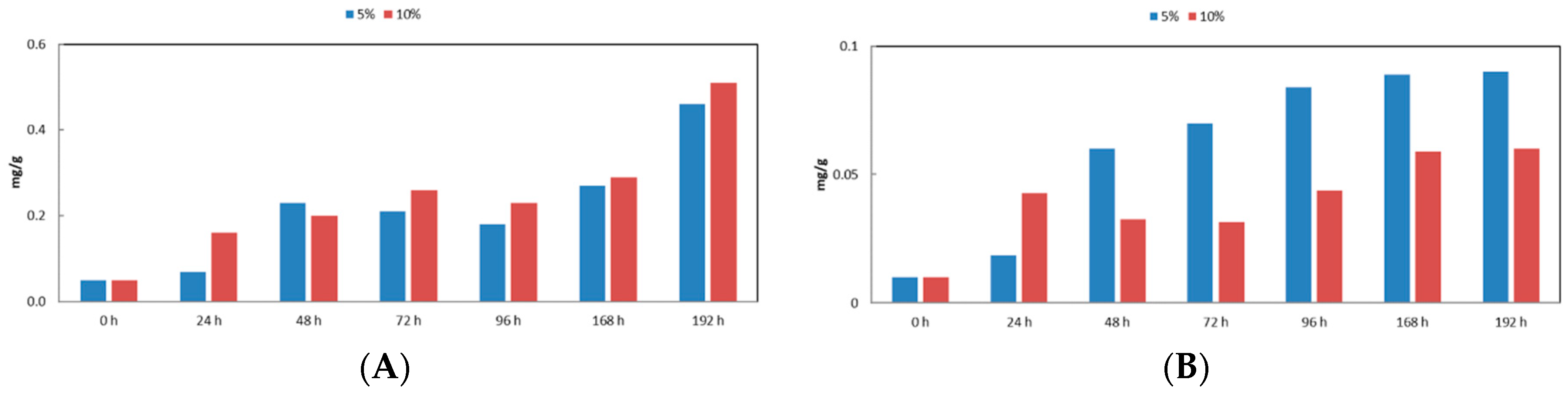
3. Results and Discussion
4. Conclusions
- Culture media to grow microalgae C. sorokiniana using by-products from the wine industry has been optimized, extracting with distilled water at room temperature the soluble nutrients contained in wine lees using them at different concentrations w/v.
- Stable cultures of the microalgae C. sorokiniana have been obtained using culture media prepared with soluble nutrients (MSN) extracted from lees as wine residues at concentrations from 5 to 30% w/v. Optimal growth was reached with MSN cultures prepared at both 5 and 10% w/v.
- At 10% oxidative stress, measured as carotenoids production (specially lutein) and antioxidant activity (DPPH method), was more intense than the obtained using residues at 5%. Our results show that growth in culture media prepared with wine lees extracts stimulated the antioxidant activity and the production of carotenoids in C. sorokiniana cells.
- A first approach in the search for new and sustainable uses of wine industry by-products in the context of a circular economy is presented as these residues could be used to obtain carotenoid-enriched microalgae biomass.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MSN | Medium prepared with Soluble Nutrients of wine by-products |
| w/v | weight/volume |
| DPPH | 1,1-diphenyl-2-picrylhydrazyl |
References
- Devesa-Rey, R.; Vecino, X.; Varela-Alende, J.L.; Barral, M.T.; Cruz, J.M.; Moldes, A.B. Valorization of winery waste vs. the costs of not recycling. Waste Manag. 2011, 31, 2327–2335. [Google Scholar] [CrossRef] [PubMed]
- Moreira, M.M.; Rodrigues, F.; Dorosh, O.; Pinto, D.; Costa, P.C.; Švarc-Gajić, J.; Delerue-Matos, C. Vine-Canes as a Source of Value-Added Compounds for Cosmetic Formulations. Molecules 2020, 25, 2969. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, A.; Nawrocka, A.; Wiącek, D.; Krzemińska, I. Agro-industrial by-product in photoheterotrophic and mixotrophic culture of Tetradesmus obliquus: Production of ω3 and ω6 essential fatty acids with biotechnological importance. Sci. Rep. 2020, 10, 6411. [Google Scholar] [CrossRef] [PubMed]
- Mandalam, R.K.; Palsson, B. Elemental balancing of biomass and medium composition enhances growth capacity in high density Chlorella vulgaris cultures. Biotechnol. Bioeng. 1998, 59, 605–611. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyll and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant. Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Ruiz-Domínguez, M.C.; Vaquero, I.; Obregón, V.; De la Morena, B.; Vílchez, C.; Vega, J.M. Lipid accumulation and antioxidant activity in the eukaryotic acidophilic microalga Coccomyxa sp. (strain onubensis) under nutrient starvation. J. Appl. Phycol. 2014, 27, 1099–1108. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. LEB 1995, 38, 25–30. [Google Scholar] [CrossRef]
- Schreiber, U.; Endo, T.; Mi, H.; Asada, K. Quenching analysis of chlorophyll fluorescence by the saturation pulse method: Particular aspects relating to the study of eukaryotic algae and cyanobacteria. Plant Cell Physiol. 1995, 36, 873–882. [Google Scholar] [CrossRef]
- Forján, E.; Vílchez, C.; Vega, J.M. Biotecnología de Microalgas; CEPSA: Huelva, Spain, 2014; pp. H201–H214. [Google Scholar]
- Marxen, K.; Vanselow, K.H.; Lippemeier, S.; Hintze, R.; Ruser, A.; Hansen, U.-P. Determination of DPPH radical oxidation caused by methanolic extracts of some microalgal species by linear regression analysis of spectrophotometric measurements. Sensors 2007, 7, 2080–2095. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martin-Gordillo, L.; Cuaresma, M.; Fernández-Recamales, M.Á.; Sayago, A.; Vílchez, C.; Garbayo, I. Cultivation of Microalgae Chlorella Using Wine Industry by-Products. Proceedings 2020, 66, 30. https://doi.org/10.3390/proceedings2020066030
Martin-Gordillo L, Cuaresma M, Fernández-Recamales MÁ, Sayago A, Vílchez C, Garbayo I. Cultivation of Microalgae Chlorella Using Wine Industry by-Products. Proceedings. 2020; 66(1):30. https://doi.org/10.3390/proceedings2020066030
Chicago/Turabian StyleMartin-Gordillo, Lidia, María Cuaresma, Mª Ángeles Fernández-Recamales, Ana Sayago, Carlos Vílchez, and Inés Garbayo. 2020. "Cultivation of Microalgae Chlorella Using Wine Industry by-Products" Proceedings 66, no. 1: 30. https://doi.org/10.3390/proceedings2020066030
APA StyleMartin-Gordillo, L., Cuaresma, M., Fernández-Recamales, M. Á., Sayago, A., Vílchez, C., & Garbayo, I. (2020). Cultivation of Microalgae Chlorella Using Wine Industry by-Products. Proceedings, 66(1), 30. https://doi.org/10.3390/proceedings2020066030





