Abstract
The presence of TiO2 nanoparticles in a cementitious matrix induces self-cleaning capacity in the presence of UV radiation by combining two mechanisms: surface hydrophilicity and degradation of the stain agent molecules. Experimental results indicate an increase in surface water absorption and, indirectly, in the degree of hydrophilicity, with the increase in the concentration of TiO2 nanoparticles in the matrix. Degradation of organic molecules, rhodamine B, is dependent on the duration of action and intensity of UV rays and the concentration of nanoparticles in the cementitious matrix. An addition of 3%–6% TiO2 is effective and sufficient for a good self-cleaning capacity of cementitious surfaces.
1. Introduction
One of the most common causes of structural degradation is the accumulation of polluting materials, organic or inorganic, on the surface [1,2,3,4]. Nanotechnology could offer a solution by producing materials with self-cleaning capacities. A cementitious composite containing TiO2 nanoparticles, under the influence of UV rays, has a specific behavior: a photocatalysis of redox reactions is manifested that allow the oxidation and decomposition of organic particles on the cementitious surface (particles that produce the staining effect) into smaller particles, molecules with a simpler structure, which can either be more easily taken up by rainwater and removed (washing phenomenon).On the one hand, the organic particles continue to decompose until the final reduction (Equation (1)) [5,6,7,8,9,10,11,12,13,14]; on the other hand, the hydrophilicity of the cement composite surface increases (Figure 1). The two mechanisms together realize the self-cleaning capacity of cementitious composites containing TiO2 nanoparticles [1,15,16,17,18,19,20,21,22]. According to the literature [14], TiO2 nanoparticles, alone, lose their property of influencing hydrophilicity and oxidation of organic molecules as soon as the UV action ceases, but in combination with SiO2 in cement, this photoactivated capacity is prolonged even with more days of darkness (total lack of UV radiation).
Organic compound + O2 → TiO2, hυ → CO2 + H2O + Residual inorganic compound
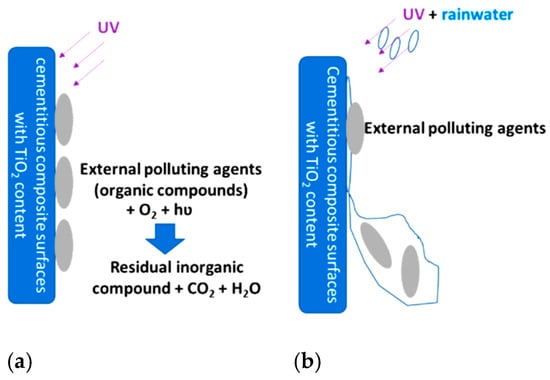
Figure 1.
Schematic representation of the self-cleaning capacity by combining the two mechanisms: (a) photocatalytic oxidation process of organic substances; (b) removal of foreign substances with the help of rainwater by increasing surface hydrophilicity.
The mechanism underlying this property of cementitious composite surfaces containing TiO2 nanoparticles, under conditions of exposure to the action of UV rays, can be explained by the increase of hydroxyl groups (OH−), a phenomenon identified by X-ray photoemission spectroscopy (XPS), Fourier-transform infrared spectroscopy (FTIR) or nuclear magnetic resonance (NMR), and shown schematically in Figure 2 [15,23,24,25,26]. The transition of the surface, under the influence of UV radiation, into a metastable thermodynamic state is the result of the coexistence of the forms of molecular water and dissociated water. Under the action of UV rays, titanium dioxide is a semiconductor with a band gap of about 3.0 eV that in the presence of UV light, through energy absorption, generates electrons (e−) and holes (h+) (Equation (2)). Electrons tend to reduce the cation Ti(IV) to the ion Ti(III) (Equation (3)), and voids oxidize O2− anions (Equation (4)). This process will release oxygen and thus form gaps on the surface of titanium dioxide, giving the possibility of binding water molecules with the release of hydroxyl groups (OH−). The oxidation capacity of holes (h+) is higher than the electron reduction capacity (e−); on the surface of the photocatalyst is a single layer of adsorbed H2O molecules, and hydroxyl groups are formed (OH−). These hydroxyl groups are strongly oxidizing and react with molecules of organic nature producing free radicals of peroxyl type, which will react with molecular oxygen in a chain of reactions to the final products CO2 and H2O. On the other hand, electrons (e−) reduce oxygen to the free radical O2− which will react with the peroxide molecules resulting during the reaction between hydroxyl groups and organic molecules, ultimately leading to a chain of reactions to the final products CO2 and H2O [5,6,7,8,9,10,11,12,13]. Photogenerated holes (h+) increase the length of the links within the TiO2 network (Figure 2), bringing the surface to a metastable state that allows the adsorption of molecular water, simultaneously with the formation of new hydroxyl groups and the release of a proton [15,27].
TO2 + hʋ → e− + h+
e− + Ti4+ →Ti3+
4h+ + 4O2− → 2O2
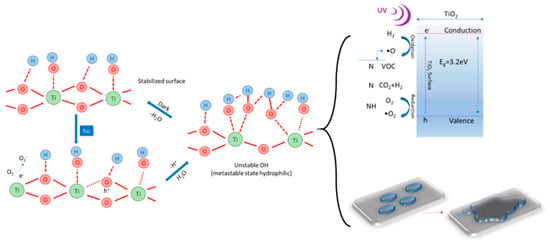
Figure 2.
Schematic representation of the transition of the surface to a metastable state by photoactivation in the presence of UV rays [13,15,26].
Regarding the hydrophilicity assessment, the literature indicates that, in principle, the two methods of measurement—direct method of measuring the contact angle of the droplet of the liquid to the surface [1,28,29] and the indirect method for the determination of the water absorption on the surface [1,14,28] which states that there is a high potential of water absorption on the surface—are indicated by a high level of wettability. To highlight the ability of self-cleaning, research in the literature indicates several test methods, of which the method of staining with rhodamine B (RhB) is the most common.
Photocatalized degradation of RhB in the presence of TiO2 is a process that is based on the formation of molecular ions RhB+• and the formation of O2−•, highly reactive species that will cause the degradation of the organic molecule [30,31,32]. The principle of this method is relatively simple: the surface of the test specimen is smeared with an aqueous solution of rhodamine B, after which it is exposed to the action of UV rays by combining or not with additional actions, for example, artificial rain. Measurable indicators expressing the degree of staining/cleaning of the surface are then identified and compared [30,33,34,35,36]. The UNI 11259 standard regulates this test by indicating the amount of aqueous solution of rhodamine B applied to the surface 0.05 g/dm2 and, as a measurable indicator, the measurement of the degree of staining [36]. In the literature, there are reports that comply with this standard, as well as reports of tests performed with deviations from UNI 11259, especially with regard to the concentration of rhodamine B solution (0.5 g/L and 1 g/L, respectively) [36].
Using the standardized method or modified methods, the results of the conducted research indicate discoloration by 20% after exposure for 1 min and by 75%–95% after exposure for 4 h to the action of UV rays [36]; the dependence of self-cleaning performance on characteristics of titanium dioxide used-granule size, granulometric distribution, type, crystalline structure and ratio of the three crystalline structures (rutile-tetragonal crystalline structure, anatase-tetragonal crystalline structure and brookite-orthorhombic crystalline structure) [30,34,35]; and non-alteration of the photocatalytic efficiency of TiO2 nanoparticles following accelerated aging cycles performed on cementitious composites [36]. After the exposure of samples to accelerated aging cycles simulating a period of five years in climatic conditions typical of northern Denmark, good behavior and durability of this property were observed [30,34]. Increased self-cleaning performance with increased TiO2 nanoparticle content in the range 0%–3% was also observed [33]. Zhang et al. [37] indicated an increase of the self-cleaning effect with the increase of the time of action of the UV rays and a long-term efficiency of this performance for cement composites with the content of up to 6% TiO2; however, they recommended maintaining the dosage of TiO2 to a maximum of 2%. They also indicated a possible reduction in performance with the increase of the irradiation duration, performances that are subsequently recovered as soon as the specimen is subjected to the action of wet-dry cycles, artificial rain and therefore the removal by the washing of reaction products from the surface [37].
Until recently it was considered that these cementitious composites are intended exclusively for outdoor use because of the need for solar radiation and the UV content to activate the TiO2 nanoparticles. Currently, research has shown that even in indoor conditions, minimum radiation of 1 µW/cm2 from a fluorescent tube is sufficient to achieve the photocatalytic effect [15].
The aim of this research was to analyze the influence of the addition of TiO2 nanoparticles in the cementitious composite matrix on the surface hydrophilicity and photochemical degradation capacity of the organic staining agent molecule, as well as to identify an optimal dosage for the TiO2 nanoparticles in the cementitious matrix, thus providing valuable information for obtaining the self-cleaning capacity as best as possible.
2. Materials and Methods
2.1. Materials
In order to carry on the tests, prismatic specimens (plates) with an exposed surface of 0.085 m2 were produced using white Portland cement (CEM I 52.5R) and Degussa P25 TiO2 nanoparticles in different ratios: 1% (P2), 2% (P3), 3% (P4), 3.6% (P5), 4% (P6), 5% (P7), 6% (P8) and 10% (P8), percentage relative to cement quantity. One mixture was prepared without the addition of TiO2 nanoparticles, which was considered the control sample (P1). For all cases, the ratio of water/dry powder material of 0.5 was maintained constant, where dry powder material represented the sum of the amounts of cement and TiO2 nanoparticles in each mixture. After mixing the materials, the test samples were kept for 28 days for aging, according to EN 196 conditions.
2.2. Hydrophilicity
The 28 days age test samples, dried until constant mass, were exposed with an inclination of 10° from the vertical. From a constant distance of 30 cm, 5 mL of distilled water were sprayed every 2 min until a cumulative volume of 50 mL was reached. After each test step (after each spray of 5 mL distilled water), the samples were weighted, and the surface water absorption was calculated. After the test, the specimens were dried until constant mass and exposed for 1 h to the action of UV rays with a wavelength in the UVA field and luminous intensity of 405 lux, after which the test was repeated. Subsequently, the samples were dried again until constant mass and exposed to UV for another 24 h, and the test for determination of surface water absorption was repeated [38].
The surface water absorption was calculated as an indirect indicator of the degree of hydrophilicity of the surface of the cementitious composite (Equation (5)):
where mt—test specimen mass at time t (2, 4, 6, 8, 10, 12, 14, 16, 18, 20 min) from the start of spraying, which corresponds to sprayers with a volume of (5, 10, 15, 20, 25, 30, 35, 40, 45, 50 mL) distilled water; m0—the initial mass of the dry specimen at constant mass; A—the surface of the specimen exposed to spraying.
Qt = (mt − m0)/A (kg/m2)
For each instance of activation by UV exposure or without UV exposure, the efficiency indicator of the addition of TiO2 in the cementitious matrix was defined on the surface water absorption property (EIN) representing the percentage variation (increase) of the total surface water absorption (time 20 min, volume of water 50 mL) of the test piece of cementitious composite with the addition of TiO2 nanoparticles, total surface water (time 20 min, water volume 50 mL) from cementitious composite without the addition of TiO2 nanoparticles (Equation (6)):
where Q20xUV—surface water absorption of the cementitious composite specimen with TiO2 nanoparticles addition at time 20 min after the start of the test (50 mL water); Qt20control sample xUV—surface water absorption of the control specimen (without TiO2 nanoparticles addition) at time 20 min after the start of the test (50 mL water); x—number of UV exposure hours (0 h, 1 h or 1 + 24 h).
EIN = (Q20xUV − Qt20control sample xUV)/Q20control sample UV · 100 (%)
Thus, indirectly, it can be said that the higher the efficiency index of nanoparticles, the more hydrophilicity increased, the cementitious mixture was more efficient, and the addition of nanoparticles was more effective in improving the performance of the composite.
2.3. Self-Cleaning Capacity
The principle of the method consisted of controlled staining, exposure to conditions of activation of the self-cleaning property and determination of the degree of whiteness of the stained surface (GA) as an indirect indicator of the self-cleaning capacity of cementitious composites containing TiO2 nanoparticles. Thus, the test samples were stained by dripping, using as stain agent an aqueous solution of rhodamine B (1 g/L), applied in equal amounts on the surface of the samples. Subsequently, the samples were subjected to a cycle of action of UV rays, water through artificial rain and drying, according to the test diagram presented in Figure 3. After each step of the test diagram, the degree of whiteness (GA) was measured, and at predetermined intervals, the stained surface of the specimen (AM) was analyzed microscopically.
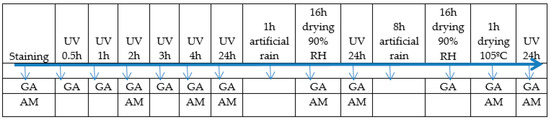
Figure 3.
Self-cleaning capacity test diagram. GA: the degree of whiteness; AM: the stained surface of the specimen (*RH - relative humidity).
Photoactivation of the surface was performed using a UV light source with emission in the spectrum 315–400 nm, corresponding to the UVA band. To assess the influence of UV radiation intensity on self-cleaning capacity, the irradiation source was placed at a distance of 10 cm and 20 cm for the second phase, above the surface of the specimens, which resulted in a luminous flux intensity of 860 lux and 405 lux, respectively. Exposure to artificial rain and drying were carried out in the absence of any light source.
The test equipment was composed of the following individual stations: UVA source exposure enclosure; artificial rain exposure enclosure; visual analysis area; microscopic analysis area and whiteness degree recording area The degree of whiteness was measured with a WSB-1 leukometer.
The efficiency of the addition of TiO2 nanoparticles on the self-cleaning character of the cementitious composite was analyzed visually and through two measurable parameters:
- Degree of the whiteness of the sample, determined in the stained area;
- Degree of the ability to recover the whiteness—represents, as a percentage, how much of the whiteness degree of the stained sample was recovered in relation to the initial whiteness degree, after going through one or more steps in the test diagram (Equation (7)):
CR = ((GAt − GA0)/GAc)∙100 (%)
On the basis of these measurable indicators, it was thus assessed that the greater the whiteness degree and the recovery capacity of the whiteness degree increase from one step of the test diagram to another, the more pronounced the self-cleaning effect.
3. Results and Discussion
3.1. Hydrophilicity
The results obtained from the point of view of the measurable parameters identified for the indirect measurement of the hydrophilicity of the surface of the cement compounds and with respect to the kinetics of the process are shown in Figure 4 and Figure 5.
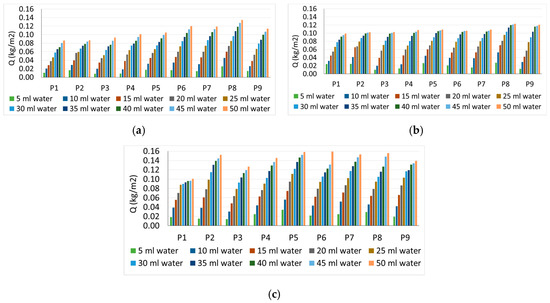
Figure 4.
Surface water absorption: (a) non-UV specimens; (b) 1 h UV specimens; (c) 1 + 24 h UV specimens.
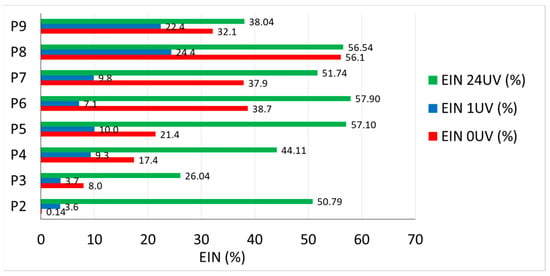
Figure 5.
Efficiency Index of Nanoparticles (EIN): non-UV test specimens (red), 1 h UV test specimens (blue), 1 + 24 h UV test specimens (green).
Surface water absorption (Figure 4) increases continuously and steadily for all analyzed cases with the increase in the amount of water sprayed. This increase can be expressed mathematically by a Grade 1 equation according to the amount of water sprayed, in the form of ax+b, for each case analyzed. It is noted that the maximum water absorption is in the case of P8 composition with 6% TiO2, for all situations of the amount of water sprayed, therefore it is appreciated that this composite matrix has the highest hydrophilicity in conditions not exposed to UV, respectively exposure for 1 h to the action of UV rays. In the case of samples exposed 1 + 24 h to UV action, the maximum water absorption is initially in the case of composition P5 (3.6% TiO2), followed very closely by P8 (6% TiO2), but with the advance of the test (increasing the amount of water sprayed, there is an increase in water absorption for all specimens in the range P2–P8 (1% TiO2–6% TiO2). Increasing the amount of TiO2 over 6% causes a reduction of water absorption on the surface, which indicates a reduction of hydrophilicity (P9-10% TiO2). Sample P2 (1% TiO2) in the case of test specimens not exposed to UV action and samples P2 (1% TiO2) and P3 (2% TiO2) in the case of test specimens exposed for 1 h to UV action indicate rather small changes in hydrophilicity, compared to the control. It can thus be considered that the addition of 1%–2% TiO2 in the cementitious matrix is insufficient. The slight flattening of the curve, with the increase of the amount of water sprayed, can be considered as a measure of the tendency of stabilization of the phenomenon to achieve a maximum of the amount of water absorbed on the surface, after which the excess water has a chance to slip on the existing.
In all cases, as expected, the control sample (P1) shows a much-reduced evolution of the process of water absorption on the surface, compared to the composite samples with nanoparticles content, a sign that this surface is less hydrophilic, most of the amount of water remaining, probably, as drops that fail to form a film and fall easily.
In general, it can be said that at the beginning of the process (small amounts of water sprayed), the evolution of water absorption on the surface from one spray to the next is more strongly influenced by the amount of TiO2. As the amount of water sprayed increases, the absorption process on the surface stabilizes, the percentage of water added by each spray generally decreasing.
As seen in Figure 5, for samples tested without UV exposure and those tested with 1 h UV exposure, respectively, sample P8 (6% TiO2) shows the highest efficiency in terms of the influence of the addition of TiO2 on surface hydrophilicity compared to the control sample, but sample P9 (10% TiO2) indicates a reduced efficiency of the addition of nanoparticles on surface hydrophilicity, eventhough the quantity of nanoparticles in the matrix is the highest. In the case of test specimens tested after 1 + 24 h exposure to UV rays, samples P6 (4% TiO2) and P5 (3.6% TiO2) have the highest efficiency in terms of the influence of the addition of TiO2 on the surface hydrophilicity, compared to the control sample.
3.2. Self-Cleaning
Visually, evolution according to expectations was observed: discoloration of the stained area, both for exposure to UVA with the intensity of 860 lux and for the intensity of 405 lux, as shown, for example, for the control specimen made of the cementitious composite without TiO2 content and for the specimen made of cementitious composite with 4% TiO2 content in Figure 6 and Figure 7, respectively.

Figure 6.
Discoloration of test specimens following the test diagram, example P1—control specimen made of the cementitious composite without TiO2 content: before (a) and after (b) the test diagram, with luminous intensity 860 lux; before (c) and after (d) the test diagram, with luminous intensity 405 lux.

Figure 7.
Discoloration of test specimens following the test diagram, example P5—test specimen made of the cementitious composite with 4% TiO2 content: before (a) and after (b) the test diagram, with luminous intensity 860 lux; before (c) and after (d) the test diagram, with luminous intensity 405 lux.
The results obtained from the point of view of the measurable parameters identified for measuring the self-cleaning capacity are shown in Figure 8 and Figure 9.
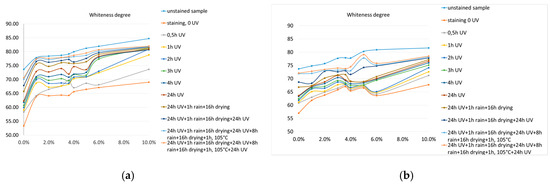
Figure 8.
Whiteness degree: (a) test diagram with the luminous intensity 860 lux; (b) test diagram with the luminous intensity 405 lux.
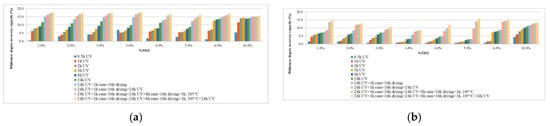
Figure 9.
Whiteness degree recovery capacity: (a) test diagram with light intensity 860 lux; (b) test diagram with light intensity 405 lux.
Immediately after staining, the degree of whiteness of the specimens is significantly reduced, as expected (Figure 8), thus obtaining a degree of staining, expressed as a percentage reduction of the degree of whiteness, within the range (18.0%–27.6%). By exposing stained specimens to the photocatalytic action of UV, the degree of whiteness increases steadily (Figure 8) with each evaluation after the preset exposure ranges (0.5 h, 1 h, 2 h, 3 h, 4 h, 24 h). Visually the discoloration of the spots is observed, and the microscopic analysis showed that there is a discoloration especially of the surface in the immediate vicinity of the micro-cracks that appeared during the ripening process. An exception is the control specimen made without the addition of TiO2 nanoparticles: in this case, only a slight discoloration is observed, a phenomenon explained, on the one hand, due to the chemical degradation of the staining agent under the influence of UV, and on the other hand, due to the existence, from the manufacturing process, of a quantity of TiO2, even if not much. Analyzing the evolution of the degree of whiteness of the test specimens throughout the course of the test diagram, a significant jump is observed, in most cases, with the introduction of the washing agent, artificial rain, both in the case of high-intensity and low-intensity UV radiation.
The evolution of the control specimen is the least satisfactory, remaining intently stained throughout many stages of the test diagram, undergoing a cleaning process, probably more mechanical by the action of water droplets, its discoloration being more evident after the last two steps of the test diagram.
From the point of view of the mathematical modeling possibility of the evolution of the degree of whiteness, depending on the amount of nanoparticles added in the cement matrices, one can identify polynomial functions of degree 3 that provide a precision rate R2 minimum 0.8.
If analyzing, from the point of view of the ability to recover the degree of whiteness (Figure 9), the behavior of cementitious matrices in the presence of UV radiation with the intensity of 860 lux, but without the action of rain, one could say that content of TiO2 nanoparticles, in relation to the amount of cement, of around 6% is desirable. With the introduction of artificial rain/drying agents into the test diagram, the behavior of the test specimens’ changes, suggesting a concentration of max. 4% TiO2 nanoparticles in the cementitious matrix. The same cannot be said about test specimens tested at an incident UV radiation of lower intensity (405 lux), where a higher concentration of TiO2 nanoparticles in the cementitious matrix would be desirable.
Tests carried out by the method of staining with rhodamine B provide evidence of the self-cleaning ability of cementitious matrices enriched with TiO2 nanoparticles by photoactivation under the influence of UVA rays. However, the experimental results obtained cannot provide the basis for a documented selection of the optimal nanoparticle content, so that the cost–benefit balance is the most favorable. It is considered, therefore, that further research is needed, as well as a correlation between the results obtained by experiments, on the one hand, with the other results reported in the literature and, on the other hand, the results obtained by experiments on the evolution of the physical-mechanical properties of the cementitious matrix based on the content of the nanoparticles, together with an analysis from the point of view of whether or not the field is intended to be used.
4. Conclusions
The aim of this experimental study was to analyze the influence of the content of nanoparticles in the cement matrix on the two mechanisms that together realize the property of self-cleaning.
After analyzing, as an indirect indicator of hydrophilicity, the surface water absorption of all specimens, tested in all three conditions of exposure to the action of UV rays, it can be said that regardless of the content of TiO2 nanoparticles, in all cases, exposure to UV increases the hydrophilicity of the surface, compared to the situation of a lack of this exposure and the increase. The satisfactory behavior of P8 samples (6% TiO2) and the unsatisfactory behavior of P9 samples (10% TiO2), as well as the behavior strongly dependent on the UV exposure conditions of cementitious matrices with a low content of TiO2 nanoparticles, can easily be observed.
From the point of view of the decomposition capacity of the organic molecule rhodamine B, it can be said that the increase of the amount of TiO2 introduced in the cementitious matrix increases the degree of whiteness but also the self-cleaning capacity of the specimens. The self-cleaning capacity is also influenced by the intensity of the incandescent UV light flux on the surface of the sample, better results being recorded for a higher UVA intensity. In the absence of the action of rain (without accumulating the effect of increasing the hydrophilicity of the surface), it can be said that in case of a lower incident UV radiation a higher concentration of nanoparticles in the cementitious matrix is desirable. This need, however, can be reduced either by increasing the intensity of UV radiation or by combining with the action of washing water (the jump of the cleaning index being significant with the first step of artificial rain in the test diagram). However, it can be assessed that, from a cost-benefit point of view, a concentration of 10% TiO2 nanoparticles is not justified because the improvement of quantifiable parameters compared to those recorded for the 6% TiO2 composition is not proportional to the consumption of nanoparticles.
Author Contributions
Conceptualization, A.H. and E.G.; methodology, A.H., E.G., H.S., A.-V.L. and B.A.I.; formal analysis, A.H., E.G., H.S., A.-V.L. and B.A.I.; investigation, A.H., E.G., H.S., A.-V.L. and B.A.I.; writing—original draft preparation, A.H. and E.G.; writing—review and editing, A.H., E.G., H.S. and A.-V.L.; visualization, A.H., E.G., H.S. and A.-V.L.; supervision, H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
Authors would like to thank Evonik Industries AG Hanau, Germany, for the donations of the necessary materials used in the current study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Graziani, L.; Quagliarini, E.; Bondioli, F.; D’Orazio, M. Durability of self-cleaning TiO2 coatings on fired clay brick façades: Effects of UV exposure and wet & dry cycles. Build. Environ. 2014, 71, 193–203. [Google Scholar]
- Cucek, L.; Klemes, J.J.; Kravanja, Z. A review of footprint analysis tools for monitoring impacts on sustainability. J. Clean. Prod. 2012, 34, 9–20. [Google Scholar] [CrossRef]
- Chiarini, A. Designing an environmental sustainable supply chain through ISO 14001 standard. Manag. Environ. Qual. Int. J. 2012, 24, 16–33. [Google Scholar] [CrossRef]
- Chiarini, A. Strategies for developing an environmentally sustainable supply chain: Differences between manufacturing and service sectors. Bus. Strat. Environ. 2014, 23, 493–504. [Google Scholar] [CrossRef]
- Fox, M.A.; Chen, C.C. Mechanistic features of the semiconductor photocatalyzed olefin-to-carbonyl oxidative cleavage. J. Am. Chem. Soc. 1981, 103, 6757–6759. [Google Scholar] [CrossRef]
- Cunningham, J.; Srijaranai, S. Isotope-effect evidence for hydroxyl radical involvement in alcohol photo-oxidation sensitized by TiO2 in aqueous suspension. J. Photochem. Photobiol. A Chem. 1988, 43, 329–335. [Google Scholar] [CrossRef]
- Brezová, V.; Stasko, A.; Lapcik, L., Jr. Electron paramagnetic resonance study of photogenerated radicals in titanium dioxide powder and its aqueous suspensions. J. Photochem. Photobiol. A Chem. 1991, 59, 59–115. [Google Scholar] [CrossRef]
- Kamat, P.V. Photochemistry on nonreactive and reactive (semiconductor) surfaces. Chem. Rev. 1993, 93, 267–300. [Google Scholar] [CrossRef]
- Courbon, H.; Formenti, M.; Pichat, P.J. Study of oxygen isotopic exchange over ultraviolet irradiated anatase samples and comparison with the photooxidation of isobutane into acetone. Phys. Chem. 1977, 81, 550–554. [Google Scholar] [CrossRef]
- Anpo, M.; Chiba, K.; Tomonari, M.; Coluccia, S.; Che, M.; Fox, M.A. Photocatalysis on Native and Platinum-Loaded TiO2 and ZnO Catalysts —Origin of Different Reactivities on Wet and Dry Metal Oxides. Bull. Chem. Soc. Jpn. 1991, 64, 543–551. [Google Scholar] [CrossRef]
- Sun, L.; Bolton, J.R. Determination of the Quantum Yield for the Photochemical Generation of Hydroxyl Radicals in TiO2 Suspensions. J. Phys. Chem. 1996, 100, 4127–4134. [Google Scholar] [CrossRef]
- Grela, M.A.; Coronel, M.E.J.; Colussi, A.J. Quantitative Spin-Trapping Studies of Weakly Illuminated Titanium Dioxide Sols. Implications for the Mechanism of Photocatalysis. J. Phys. Chem. 1996, 100, 16940–16946. [Google Scholar] [CrossRef]
- Fujishima, A.; Rao, T.N.; Tryk, D.A. Titanium dioxide photocatalysis. J. Photochem. 2000, 1, 1–21. [Google Scholar] [CrossRef]
- Quagliarini, E.; Bondioli, F.; Goffredo, G.B.; Cordoni, C.; Munafo, P. Self-cleaning and de-polluting stone surfaces: TiO2 nanoparticles for limestone. Constr. Build. Mater. 2012, 37, 51–57. [Google Scholar] [CrossRef]
- Irie, H.; Hashimoto, K. Photocatalytic Active Surfaces and Photo-Induced High Hydrophilicity/High Hydrophobicity. Hdb. Environ. Chem. 2005, 2, 425–450. [Google Scholar]
- Chen, J.; Poon, C.-S. Photocatalytic construction and building materials: From fundamentals to applications. Build. Environ. 2009, 44, 1899–1906. [Google Scholar] [CrossRef]
- Fujishima, A.; Zhang, X. Titanium dioxide photocatalysis: Present situation and future ap-proaches. CR Chim. 2006, 9, 750–760. [Google Scholar] [CrossRef]
- Guan, K. Relationship between photocatalytic activity, hydrophilicity and self-cleaning effect of TiO2/SiO2 films. Surf. Coat. Technol. 2005, 191, 155–160. [Google Scholar] [CrossRef]
- Lee, Y.C.; Hong, Y.P.; Lee, H.Y.; Kim, H.; Jung, Y.J.; Ko, K.H. Photocatalysis and hydrophilicity of doped TiO2 thin films. J. Colloid Interf. Sci. 2003, 267, 127–131. [Google Scholar] [CrossRef]
- Graziani, L.; Quagliarini, E.; Osimani, A.; Aquilanti, L.; Clementi, F.; Yéprémian, C. Evaluation of inhibitory effect of TiO2 nanocoatings against microalgal growth on clay brick façades under weak UV exposure conditions. Build. Environ. 2013, 64, 38–45. [Google Scholar] [CrossRef]
- Li, C.; Chang, S.J.; Tai, M.Y. Surface chemistry and dispersion property of TiO2 nanoparticles. J. Am. Ceram. Soc. 2010, 93, 4008–4010. [Google Scholar] [CrossRef]
- Yu, J.; Low, J.; Wei, X. Enhanced photocatalytic CO2-reduction activity of anatase TiO2 by Co-exposed {001} and {101} facets. J. Am. Chem. Soc. 2014, 136, 8839–8842. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Hashimoto, K.; Fujishima, A.; Chikuni, M.; Kojima, E.; Kitamura, A.; Shimohigoshi, M.; Watanabe, T. Photogeneration of Highly Amphiphilic TiO2 Surfaces. Adv. Mater. 1998, 10, 135–138. [Google Scholar] [CrossRef]
- Wang, R.; Sakai, N.; Fujishima, A.; Watanabe, T.; Hashimoto, K.J. Studies of Surface Wettability Conversion on TiO2 Single-Crystal Surfaces. Phys. Chem. B. 1999, 103, 2188–2194. [Google Scholar] [CrossRef]
- Nosaka, A.Y.; Kojima, E.; Fujiwara, T.; Yagi, H.; Akutsu, H.; Nosaka, Y.J. Photoinduced Changes of Adsorbed Water on a TiO2 Photocatalytic Film As Studied by 1H NMR Spectroscopy. Phys. Chem. B 2003, 107, 12042–12044. [Google Scholar] [CrossRef]
- Shen, W.; Zhang, C.; Li, Q.; Zhang, W.; Cao, L.; Ye, J. Preparation of titanium dioxide nano particle modified photocatalytic self-cleaning concrete. J. Clean. Prod. 2015, 87, 762–765. [Google Scholar] [CrossRef]
- Sakai, N.; Fujishima, A.; Watanabe, T.; Hashimoto, K.J. Enhancement of the Photoinduced Hydrophilic Conversion Rate of TiO2 Film Electrode Surfaces by Anodic Polarization. Phys. Chem. B 2001, 105, 3023–3026. [Google Scholar] [CrossRef]
- Aslanidou, D.; Karapanagiotis, I.; Lampakis, D. Waterborne Superhydrophobic and Superoleophobic Coatings for the Protection of Marble and Sandstone. Materials 2018, 11, 585. [Google Scholar] [CrossRef]
- Manoudis, P.N.; Tsakalof, A.; Karapanagiotis, I.; Zuburtikudis, I.; Panayiotou, C. Fabrication of super-hydrophobic surfaces for enhanced stone protection. Surf. Coat. Technol. 2009, 203, 1322–1328. [Google Scholar] [CrossRef]
- Folli, A.; Jakobsen, U.H.; Guerrini, G.L.; Macphee, D.E. Rhodamine B Discolouration on TiO2 in the Cement Environment: A Look at Fundamental Aspects of the Self-cleaning Effect in Concrete. J. Adv. Oxid. Technol. 2009, 12, 126–133. [Google Scholar] [CrossRef]
- Wu, T.; Liu, G.; Zhao, J.; Hidaka, H.; Serpone, N.J. Photoassisted degradation of dye pollutants. V. Self-photosensitized oxidative transformation of rhodamine B under visible light irradiation in aqueous TiO2 dispersions. Phys. Chem. B. 1998, 102, 5845–5851. [Google Scholar] [CrossRef]
- Chen, F.; Zhao, J.; Hidaka, H. Highly selective deethylation of rhodamine B: Adsorption and photooxidation pathways of the dye on the TiO2/SiO2 composite photocatalyst. Int. J. Photoenergy 2003, 5, 209–217. [Google Scholar] [CrossRef]
- Khataee, R.; Heydari, V.; Moradkhannejhad, L.; Safarpour, M.; Joo, S.W. Self-Cleaning and Mechanical Properties of Modified White Cement with Nanostructured TiO2. J. Nanosci. Nanotechnol. 2013, 13, 5109–5114. [Google Scholar] [CrossRef] [PubMed]
- Folli, A. TiO2 Photocatalysis in Portland Cement Systems: Fundamentals of Self-Cleaning Effect and Air Pollution Mitigation. Ph.D. Thesis, University of Aberdeen, Department of Chemistry, Aberdeen, UK, 2010. [Google Scholar]
- Bianchi, C.L.; Gatto, S.; Nucci, S.; Cerrato, G.; Capucci, V. Self-cleaning measurements on tiles manufactured with microsized photoactive TiO2. Adv. Mater. Res. 2013, 2, 1–10. [Google Scholar] [CrossRef]
- Carmona-Quiroga, P.M.; Martínez-Ramírez, S.; Viles, H.A. Efficiency and durability of a self-cleaning coating on concrete and stones under both natural and artificial ageing trials. Appl. Surf. Sci. 2018, 433, 312–320. [Google Scholar] [CrossRef]
- Zhang, S.M.-H.; Tanadi, D.; Li, W. Effect of photocatalyst TiO2 on workability, strength, and self-cleaning efficiency of mortars for applications in tropical environment. In Proceedings of the 35th Conference on Our World in Concrete & Structures, Singapore, 25–27 August 2010; Available online: http://cipremier.com/100035009 (accessed on 8 February 2020).
- Hegyi, A.; Szilagyi, H.; Grebenişan, E.; Sandu, A.V.; Lăzărescu, A.-V.; Romila, C. Influence of TiO2 Nanoparticles Addition on the Hydrophilicity of Cementitious Composites Surfaces. Appl. Sci. 2020, 10, 4501. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).