1. Introduction
Sustainable development was introduced in a widespread way by the Brundtland Commission, which defined it as development that “meets the needs of the present without compromising the ability of future generations to meet their own needs” [
1]. Sustainability has been applied to many fields, including engineering, manufacturing, and design. Manufacturers are becoming increasingly concerned about the issue of sustainability. For instance, recognition of the relationship between manufacturing operations and the natural environment has become an important factor in the decision making among industrial societies [
2].
Sustainable manufacturing focuses on both how the product is made as well as the product’s attributes. This includes the inputs, the manufacturing processes, and the product’s design. Sustainable manufacturing includes things such as making products using less energy and materials, producing less waste, and using fewer hazardous materials as well as products that have greener attributes such as recyclability or lower energy use [
3].
The European steel industry generates an estimated 500,000 tones/yr of oily sludge and mill scales. More than 30% of this total is not valorised. The steelmaking by-products such as dust and mill scale are currently produced in large quantities and represent a potential of almost 5 million tons in the world [
4,
5].
Mill scale is a steelmaking by-product from the rolling mill in the steel hot rolling process. Mill scale can be considered a valuable metallurgical raw material for iron making, steelmaking, and construction industries because it contains valuable metallic minerals [
6,
7,
8].
The chemical and mineralogical characterizations of steel mill scale play a key role for their reuse in sustainable industrial applications.
The aim of this paper is the assessment of the reuse potential of the steel mill scale for sustainable application, both in the steelmaking and building materials industries.
The objectives of the paper are:
The chemical characterization of the steel mill scale;
The mineralogical characterization of the steel mill scale;
Identification of the mineralogical phases of the steel mill scale;
Establishing the mineralogical phases of the steel mill scale that are also found in cement;
Assessment of the steel mill scale potential for sustainable industrial applications.
2. Materials and Methods
The steel mill scale sample was taken from a metallurgical plant (Salaj County, Romania). The mill scale comes from the rolling process of the steel pipes. In order to identify the possibilities of reusing the mill scale, for sustainable industrial applications, the sample was subjected to chemical and mineralogical characterization. The chemical elements from the steel mill scale sample were determined using inductively coupled plasma. The mineralogical characterization of the mill scale sample was performed with the help of an X-ray diffractometer, Brucker Advance D8 type (Germany). The identification of the mineralogical phases was made with Match software from Crystal Impact. This software uses the PDF database from International Centre for Diffraction Data.
3. Results and Discussions
Table 1 shows the major and minor chemical elements contained in the steel mill scale.
According to the results of the chemical analysis, the major elements in the steel mill scale composition are iron, aluminum, silicon, magnesium, and manganese. The main constituent of the mill scale is iron with 76.8%. Due to its high iron content, the steel mill scale can be reused as a source of raw material in the sustainable steelmaking industry. According to the reference [
9], the reuse of iron, from the steel mill scale, as a raw or auxiliary material to the steelmaking, leads to natural resources conservation. The minor elements contained in the steel mill scale are chromium, nickel, molybdenum, copper, zinc, vanadium, cadmium, calcium, and, arsenic, etc. According to the references [
9,
10,
11], the chromium, nickel, molybdenum, manganese, and vanadium can be reused as alloying elements to the stainless steelmaking in the electric arc furnace.
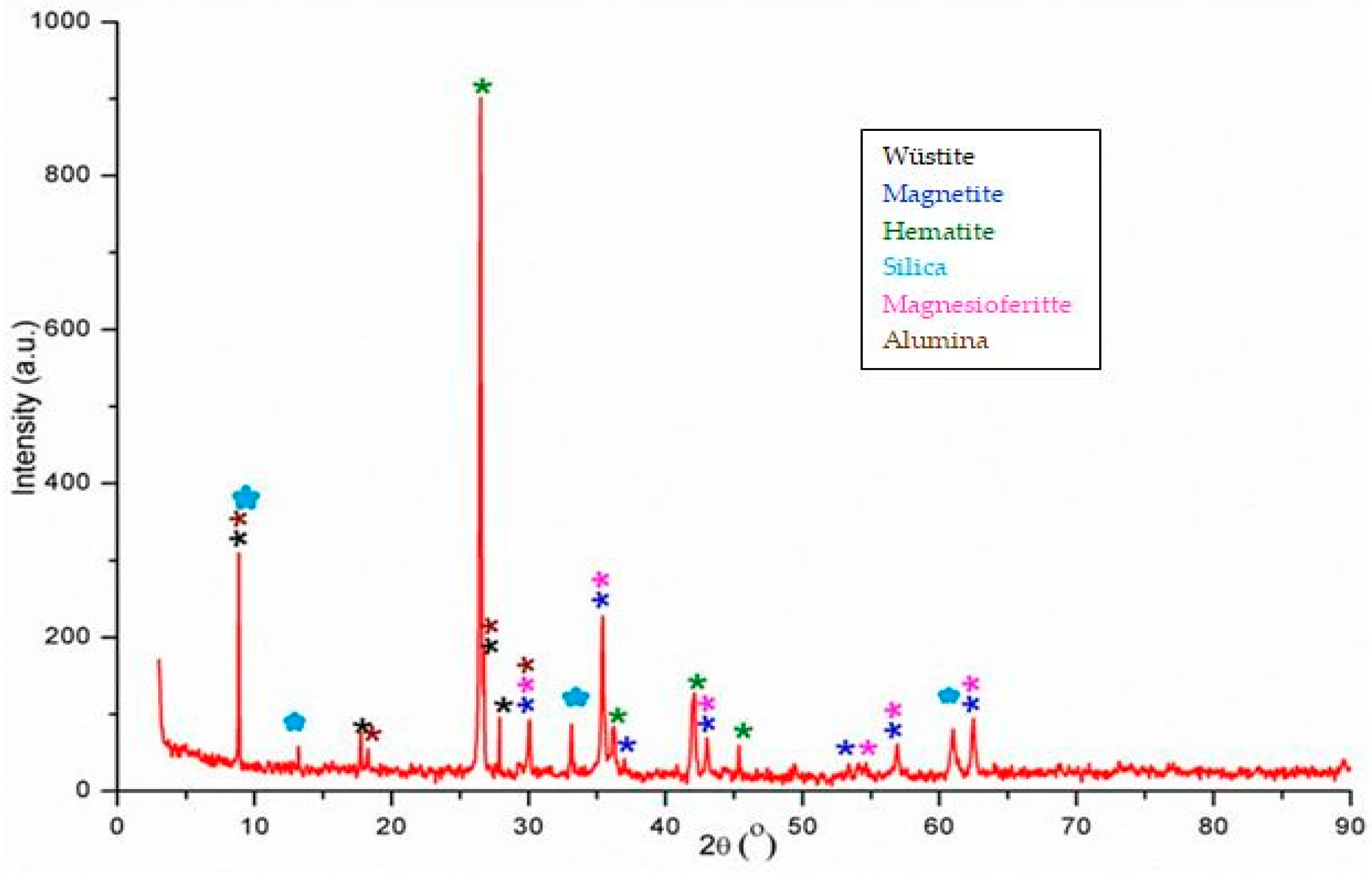
Figure 1 shows the diffractogram of the steel mill scale.
Table 2 shows the chemical formulas and powder diffraction files of the mineralogical phases identified in the steel mill scale sample, by X-ray diffraction.
According to the data presented in the
Figure 1 and
Table 2, the mineralogical phases identified in the steel mill scale are: wüstite (FeO), hematite (Fe
2O
3), magnetite (Fe
3O
4), silica (quartz) (SiO
2), magnesioferitte (MgFe
2O
4), and aluminum oxide (corundum) (Al
2O
3).
The results of the X-ray diffraction show that the mineralogical phases identified in the steel mill scale are also found in the mineralogical composition of the Portland cement. According to the references [
12,
13], the Portland cement consists mainly of lime (CaO), silica (SiO
2), alumina (Al
2O
3), and iron oxide (Fe
2O
3). In conclusion, silica, alumina, and hematite are the main mineralogical phases both in steel mill scale and in the Portland cement.
Silica, alumina, and hematite are the main compounds of the cement and contribute to the formation of the dicalcium silicate (2CaO·SiO
2), tricalcium silicate (3CaO·SiO
2), tricalcium aluminate (3CaO·Al
2O
3), and tetracalcium aluminoferrite (4CaO·Al
2O
3·Fe
2O
3). According to the references [
8,
14], the steel mill scale can be reused in the cement and mortar compositions.
The mineralogical composition of the steel mill scale plays a key role in establishing the reuse domains.
4. Conclusions
The main constituent of the mill scale is iron with 76.8%. Due to its high iron content, the steel mill scale can be reused as a source of raw material in the sustainable steelmaking industry.
The mineralogical phases identified in the steel mill scale are: wüstite (FeO), hematite (Fe2O3), magnetite (Fe3O4), silica (SiO2), magnesioferitte (MgFe2O4), and alumina (Al2O3). Silica, alumina, and hematite are the main mineralogical phases both in steel mill scale and in the Portland cement. These mineralogical phases contribute to the formation of the: dicalcium silicate, tricalcium silicate, tricalcium aluminate, and tetracalcium aluminoferrite. The results of the paper are promising and encourage the future research for establishing the optimal percentage for the reuse of the steel mill scale in the sustainable building materials.
Author Contributions
Conceptualization D.-A.I.-V., M.T., C.A. and I.-M.S.-B.; methodology D.-A.I.-V.; investigation D.-A.I.-V. and C.A.; writing—original draft preparation D.-A.I.-V., M.T., C.A. and I.-M.S.-B.; writing—review and editing D.-A.I.-V., M.T., C.A. and I.-M.S.-B.; visualization D.-A.I.-V.; supervision D.-A.I.-V.; project administration D.-A.I.-V.; funding acquisition D.-A.I.-V. All authors have read and agreed to the published version of the manuscript.
Funding
We acknowledge support by the Technical University of Cluj-Napoca.
Acknowledgments
This paper is written within the TUCN Internal Research Project Competition 2016 “Research concerning the characterization of the oily mill scale in order to identify an optimum method for reduction of the quantities of hazardous wastes landfilled”, internal competition for Research/Development/Innovation—Project 16362/07.07.2016, C.I. type 1.1-T4, Technical University of Cluj-Napoca (2016). The Internal Research Project Competition is funded by the Technical University of Cluj-Napoca in order to support the internal accredited research structures.
Conflicts of Interest
The authors declare no conflict of interest.
References
- World Commission on Environment and Development (WCED). Our Common Future; Oxford University Press: Oxford, UK; New York, NY, USA, 1987. [Google Scholar]
- Rosen, M.A.; Kishawy, H.A. Sustainable Manufacturing and Design: Concepts, Practices and Needs. Sustainability 2012, 4, 154–174. [Google Scholar] [CrossRef]
- Moldavska, A.; Welo, T. The concept of sustainable manufacturing and its definition: A content—Analysis based literature review. J. Clean. Prod. 2017, 166, 744–755. [Google Scholar] [CrossRef]
- Houbart, M. PLD-Erection of a Demonstrative De-Oiling Plant for Recycling Oily Steelmaking Sludge and Mill Scales; LIFE11 ENV/LU/000855; Layman Report: Luxembourg, 2017. [Google Scholar]
- Bienvenu, Y.; Rodrigues, S. Manufacture of Metal Powders from Pulverulent Waste, ENSMP; Centre des Matériaux, CNRS UMR 7633: Corbeil-Essonnes, France, 2007. [Google Scholar]
- Saberifar, S.; Jafari, F.; Kardi, H.; Jafarzadeh, M.A.; Mousavi, S.A. Recycling evaluation of mill scale in electric arc furnace. J. Adv. Mater. Process. 2014, 2, 73–78. [Google Scholar]
- Cartwright, D.; Clayton, J. Recycling oily mill scale and dust by injection into the EAF. Steel Times Int. 2000, 24, 42–43. [Google Scholar]
- Murthy, Y.; Agarwal, A.; Pandey, A. Characterization of mill scale for potential application in construction industry. Indian J. Eng. 2017, 35, 71–76. [Google Scholar]
- Iluțiu-Varvara, D.A.; Aciu, C.; Picӑ, E.M.; Sava, C. Research on the chemical characterization of the oily mill scale for natural resources conservation. Procedia Eng. 2017, 181, 439–443. [Google Scholar] [CrossRef]
- Yang, Q.; Holmberg, N.; Bjorkman, B. EAF smelting trials of briquettes at Avesta works of Outokumpu stainless AB for recycling oily mill scale sludge from stainless steel production. Steel Res. Int. 2009, 80, 422–428. [Google Scholar] [CrossRef]
- Iluţiu-Varvara, D.A.; Brânduşan, L.; Arghir, G.; Pică, E.M. Researches about the characterization of metallurgical slags for landfilled wastes minimization. Environ. Eng. Manag. J. 2015, 14, 2115–2126. [Google Scholar] [CrossRef]
- Soroka, I. Chemical and mineralogical composition. In Portland Cement Paste and Concrete; Palgrave: London, UK, 1979. [Google Scholar]
- Lea, F.M. The Chemistry of Cement and Concrete; Edward Arnold: London, UK, 1970; p. 158. [Google Scholar]
- Iluțiu-Varvara, D.A.; Aciu, C.; Tintelecan, M.; Sas-Boca, I.M. Assessment of recycling potential of the steel mill scale in the composition of mortars for sustainable manufacturing. Procedia Manuf. 2020, 46, 131–135. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).