Abstract
The design and synthesis of pharmaceutical cocrystals have received great interest in the recent years. Cocrystallization of drug substances offers a tremendous opportunity for the development of new drug products with superior physical and pharmacological properties such as solubility, stability, hydroscopicity, dissolution rates and bioavailability. This short review summarizes this highly topical field, covering why the topic is of interest in pharmaceutical formulation, the definitions and practical scope of cocrystals, cocrystal preparation and characterization, a comparison of different (traditional and novel) methods for cocrystal formation and the implications for regulatory control and intellectual property protection. Traditionally, cocrystals can be prepared by solvent evaporation method, grinding, and slurry method, but every method has its limitations for certain conditions. The current trend for cocrystal formation uses sophisticated methods such as the hot melt extrusion method, spray-drying method, supercritical fluid technology and the newest method: laser irradiation. The purpose of the development of a new method is not only to overcome the limitation of traditional cocrystallization methods, but also to generate simpler steps and a continuous process for the production of the cocrystal product. This article provides a brief explanation of each method that can be used to generate pharmaceutical cocrystals as well as evaluation of cocrystals. This article also covers how the developing field of cocrystallization may impact the pharmaceutical intellectual property landscape.
1. Introduction
Solubility and dissolution rates are important factors in determining the efficacy and activity of a drug. Early drug discovery studies were based on traditional remedies or serendipitous discoveries. The past two decades, however, have demanded a rational design and synthesis of drugs as a result of the emergence of new diseases and the development of drug. Many new drug targets have been identified and potential drug molecules have been synthesized and analyzed for efficacy, employing advanced techniques such as high-throughput screening and combinatorial chemistry. The lead molecules discovered utilizing these screens are increasingly large and lipophilic in nature. Therefore, the need of today’s era is to decrease problems regarding the solubility and permeability of lipophilic drugs by using different methods. Various approaches have been described by the researchers to enhance the solubility of APIs such as by formation of prodrugs, solid dispersions, size reduction, inclusion complexes with cyclodextrins, salt formation, self-emulsifying formulations, the use of surfactants, polymorphs, nanoparticles and the use of multicomponent molecular crystals. All the above techniques have their own merits and demerits but rate of success will always depend on the specific physicochemical properties of the APIs and polymers. Physical modification often used to enlarge the particle surface area, improve solubility and/or wetting of the powder and improving the stability of an API. Multi-component crystals such as solvates, hydrates, cocrystals and salts play a key role in the design of new solids, mainly in the pharmaceutical area. The poorly water-soluble drugs can be formulated as amorphous forms, crystalline solid formulations, or by lipid formulations to improve their solubility. Crystal engineering through cocrystallization is a promising approach to address problems associated with the drug [1,2,3,4].
1.1. Cocrystals:
According to the definition by the US Food and Drug Administration (FDA), cocrystals are crystalline materials composed of two or more different molecules, typically drug and cocrystal formers (“coformers”), in the same crystal lattice. Pharmaceutical cocrystals have presented opportunities for engineering solid state forms beyond conventional solid-state forms of an active pharmaceutical ingredient (API), such as salts and polymorph. This includes modification of drugs to alter physical properties of a drug, especially a drug’s solubility, without altering its pharmacology effect. The FDA also added that cocrystals can be tailored to enhance drug product bioavailability and stability so enhance the processability of APIs during drug product manufacture. As per literature, the first cocrystal synthesized was quinhydrone, which is a 1:1 cocrystal between benzoquinone and hydroquinone [5,6].
1.2. Comparison of Cocrystal and Salt
Cocrystallization has provided the pharmaceutical industry at least two advantages in as compared to salt formation: (1) According to the concept of cocrystallization, all types of molecules can form cocrystals, including weakly ionizable and non-ionizable APIs, which is considered to be a better technique in the optimization of the physical properties because salt formation is either limited or has no scope at all in such APIs. (2) In the case of salt formation due to toxicological reasons, only acidic or basic counter-ions are explored in a typical API exist salt form, whereas in the case of cocrystal screening, there is a wide range of potential cocrystal coformers which are free from toxicological constraints. The US Food and Drug Administration has maintained a list of substances (i.e., the FDA’s “generally recognized as safe” (GRAS) list: a list of substances generally recognized as safe), the contents of which number in thousands and widely used as potential co-formers for pharmaceutical cocrystals. Unlike polymorphs, which contain only one API in the crystal lattice, cocrystals are composed of an API with a neutral molecule (coformer compound) in the crystal lattice [7,8].
1.3. Importance and Design of Pharmaceutical Cocrystal
Cocrystals are important because the cocrystal solid can be designed to have superior physical properties to either of the pure starting molecules. Physical property improvement via cocrystal formation has been reported for many agrochemicals, pigments, solid explosives and particularly for pharmaceuticals. Physical property improvement is of particular interest to pharmaceuticals as the majority of medicines are delivered as solid forms. The physical properties of the solids contained within a pharmaceutical drug product will have a direct effect on the processing, delivery and finally performance of the product. To provide a best example, crystal structure directly affects the solubility of a given solid in solution. Drug products require a certain solubility to be bioavailable in the body. Most of the drugs are administered orally in solid form (80%), which is generally considered convenient and usually the safest dosage form. About 40% of them have low solubility; in fact, nearly 80–90% of drug candidates in the research and development area (R&D) pipeline have problems with low solubility, which is alarming condition for today’s era and could lead to the failure of these drugs in clinical trials [9,10].
Cocrystal formation with a suitable coformer offers the potential of improved solubility via modification of the underlying crystal structure, thus potentially rendering the compound bioavailable. As cocrystal research has expanded, it has available a range of application areas for physical property manipulation through cocrystal formation. Improvements in solubility, stability, bioavailability, dissolution rate, melting point, hydroscopicity, compressibility, bulk density, friability and mechanical properties have been well documented and emerging applications such as taste masking and intellectual property extension are being explored. The main advantages of cocrystals used to modify the properties of drugs are: (1) the molecular structure of the drug is unaltered, and it is the coformer which is the property modifying component; (2) the principles of crystal engineering and supramolecular synthons serve as a guide to control the crystal structure; (3) with a library of synthons and coformers, together with the grammar of heterosynthon hierarchy, it is possible to dial in the desired functional properties for a large number of APIs. [11,12].
The pharmaceutical cocrystals (PCs) have given a new paradigm in the solid-state modification, the utility of which the pharmaceutical industry is making serious efforts. As cocrystal research has expanded, wide range of application areas for physical property manipulation through cocrystal formation are open. Research in cocrystal structure and applications has shown an exponential increase in the last decade, evident in the number of cocrystal structures deposited in the Cambridge Structural Database and cocrystal related patent applications. Considering this, it is surprising that cocrystal preparation methods have remained, until relatively recently, largely poorly defined. Limited research attention has been directed specifically towards Cocrystal preparation. In this review, various techniques which are often utilized for cocrystallization are discussed, including an array of solid state, mechano-chemical- and liquid-assisted techniques. Additionally, several novel methodologies such as freeze-drying, micro fluidic and ultrasound-assisted cocrystallization are explained for their potential in cocrystal synthesis [13,14,15].
1.4. Design of Cocrystals:
Different theoretical approaches such as hydrogen bonding propensity, Cambridge Structure Database, supramolecular synthon, pKa values and Hansen solubility parameters have been explained for mechanism of cocrystals. The crystal engineering experiment usually involves the Cambridge Structural information (CSD) survey followed by experimental work. Cocrystals designed on the principal of the supramolecular synthesis provide a strong approach for the discovery of novel pharmaceutical solid phases. Cocrystals incorporate multiple elements in a given ratio with quantitative relation, wherever completely different molecular species move by chemical element bonding and by non-hydrogen bonding. The use of chemical element bonding rules, synthons and graph sets could help with the style and analysis of cocrystal systems. Normally, prediction of whether or not cocrystallization can occur is not, however, attainable and should, at present, be answered through empirical observation. Cocrystal formation could also be rationalized by equally considering the bond donors and acceptors of the materials in that area to be crystallized and the way they may move [16].
The two most important reasons underlying the rapid success of cocrystallization as a method of constructing advanced materials can be identified as (i) the ability to construct cocrystals following a simple design based on supramolecular synthons and (ii) the modularity of the design, that allows the exchange of cocrystal components with the intention of improving a particular solid-state property. The synthon-based design of cocrystals is continuously being expanded with halogen or hydrogen-bonding functional groups and supramolecular synthons, expanding the diversity of potential cocrystals and cocrystal components.
In cocrystals, drug and coformers interact with each other by noncovalent interaction such as hydrogen bonding, Vander waals forces or p-p stacking interactions. Hydrogen bonding plays an important role and responsible for the formation of cocrystals. Functional groups present on the API interact with functional groups present on the coformer and finally they will interact with other. Carboxylic acids, amides and alcohols are the common functional groups of APIs and coformers which are involved cocrystal formation. Most common supramolecular synthons in the Crystal Engineering: Homosynthons are formed between (a) carboxylic acid dimers, (b) amide dimers, and (c) hydroxyl dimers, whereas Heterosynthon are formed between (d) carboxylic acid and amide group, (e) carboxylic acid and aromatic nitrogen/pyridine, (f) hydroxyl and cyano group, (g) alcohol and ether group, (h) carboxylic acid and hydroxyl group, (i) hydroxyl and aromatic nitrogen/pyridine in hydrogen bond formation. All good proton donors and acceptors are used in hydrogen bonding.
Cambridge Structure Database (CSD) is valuable tool to determine the intermolecular interactions in crystals. Development of Cambridge Structure Database (CSD) is importance in the structural chemistry, material sciences and life sciences, including drug discovery and development. The CSD is well curated and updated software with about 40000 new structures added each year from journals or supplementary documents, and all of the data were carefully checked for typographical errors and for scientific integrity. The CSD may be utilized to predict the stable hydrogen bonding motifs with the intention that the most robust motifs will remain intact across a family of related structures. Statistical analysis of cocrystal data on the CSD allows for research groups to apply virtual screening techniques to find appropriate cocrystal forming pairs, so cocrystals can be designed through molecular modelling, cutting both research time and experimental cost. The CSD facilitates reliable and fast retrieval, visualization and analysis of experimentally measured crystallographic data to further understand the behavior of molecules and intermolecular forces within a crystal. This gives information concerning the type of intermolecular interaction, the geometrical preferences, the directional characteristics and the types of supramolecular synthons involved.
The cocrystals or salts formation can be predicted by a proton transfer between acid and base. Salt or cocrystal formation can be predicted from pKa value of acid and a base. The salt formation generally takes place between acid and base if the value of ΔpKa [pKa (base) – pKa (acid)] is greater than 2 or 3. Smaller value (less than 0) of ΔpKa will almost result in cocrystals formation, but the intermediate value of ΔpKa between 0 and 3 was unable to give a clear-cut distinction between cocrystals and salts. A co-crystal to salt continuum exists between 0 ≤ ΔpKa ≤ 3 values.
Molecular shape and size descriptor analysis revealed that matching of molecular shapes was more important for cocrystal formation than the matching of absolute molecular dimensions. Members of both groups (S/L is small for planar molecules and M/L is small for rod shaped, while L, M and S refers to the size of the molecule) formed cocrystals more frequently with molecules from the same group than with molecules from the other group. The statistical analysis of known cocrystals in the CSD proved that the polarities and shapes descriptors would prefer the favorable interactions between similar types of molecules, while there would be no direct correlation between negative molecular surface and hydrogen bond donors and acceptors. The observed relationships may provide useful qualitative guidelines for the rational design of cocrystals.
Fabian’s method is also used for analyzing the possibility of cocrystal formation by correlating the different descriptors (such as polarity, molecular shape, size, dipole moment). Miscibility of components in solid state could predict the likelihood of cocrystal formation and this can be predicted theoretically by calculating the Hansen solubility parameters. These approaches are very effective in the selection of coformers for the API but sometimes these would be giving the unpredictable results during the formation of cocrystals.
Some physicochemical properties such as solubility, miscibility and melting point of a material can be determined with the help of solubility parameters. Coformers can be selected to cocrystallize with a drug based on knowledge of geometries and preferred orientations of existing intermolecular interactions. Etter et al. projected the rules to facilitate the deliberate style of hydrogen-bonded solids. All of the smart nucleon donors and acceptor area units are employed in chemical element bonding; the membered ring unit chemical element bonds are important for chemical element bonds, the most effective nucleon donor and acceptor remaining when unit chemical element bonds can form hydrogen bonds to one another. These observations facilitate the handling of the difficulty of the competitive bond assemblies ascertained once a specific crystalizing agent is employed. A detailed understanding of the supramolecular chemistry of the functional groups presents in a given molecule is the essential for designing the cocrystals because it facilitates the selection of the suitable cocrystal former. Therefore, by using two or more theoretical approaches would be useful for the selection of potential coformers prior to complex experimental screening, leading to greater efficiency in cocrystal screening programs. [17,18].
2. Different Strategies of Cocrystals Formation
To date, completely different strategies have been reported for the preparation of cocrystals by researchers. Few previous strategies supported the answer and grinding has been reported for the synthesis of cocrystals. Cocrystals can be prepared by solvent- and solid-based methods [19]. Differing kinds of strategies, such as solvent evaporation, crystallization technique, anti-solvent addition, suspension conversion methodology and reaction crystallization methodology have been employed (Figure 1). Recently, some new strategies used for the formation of cocrystals, such as area unit ultrasound aided methodology, the critical fluid atomization technique spray-drying technique and the hot soften extrusion technique, have begun to emerge. According to reported methods for these, still, there is little consistency in the application of different preparation methods, or even in the terminology used to describe the same details. Details such as solvent choice, concentration of the target molecule/coformer, equilibration time and the recovery process have often not been provided. This lack of consistency and information makes it difficult to repeat or compare cocrystal preparation methods and will inevitably lead to confusion for newcomers to this research area. Therefore, the objective of this review is to systematically describe all of the reported cocrystal preparation routes and applications in a single location, in an effort to standardize the progress achieved to date in this evolving area.
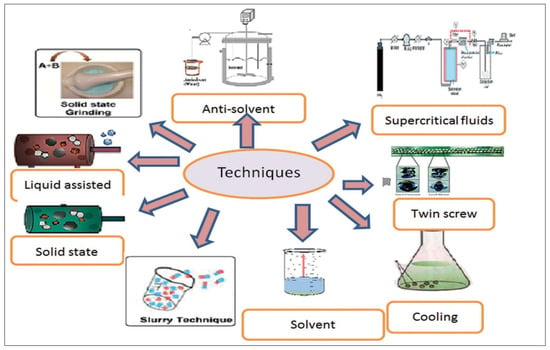
Figure 1.
Techniques of cocrystal formation (Reprinted with permission from [20]. Copyright 2015 Intech Open).
2.1. Solid State Methods
Solid-state grinding has been known for some time and a late 19th century report is often cited as the earliest reference to such a procedure, the recent technique of adding small amounts of solvent during the grinding process has been shown to enhance the kinetics and facilitate co-crystal formation and as lead to increased interest of solid state grinding as a method for co-crystal preparation. Solid state formation of pharmaceutical cocrystals has gain significant interest over the last few years due to the advantages associated with these processes. It generally includes solid phase grinding, melt extrusion and sonication (applied to either to wet or dry solid mixtures) from 80 to 85 °C. In this method, API and coformer are melted and mixed together, resulting in the cocrystal formation in a fixed stoichiometric ratio. It is basically not suitable for thermolabile moiety, but it is easy, scalable, and continuous process.
The spontaneous formation of cocrystals through the mixing of pure API and coformer under a controlled atmospheric environment condition has been reported. However, in some cases, a brief grinding of the pure components individually before mixing has been carried out. It has been reported that the cocrystallization rate in the case of premilled reactants was markedly faster than that of unmilled reactants. Moreover, higher cocrystallization rates have been reported for the same system at higher temperatures and relative humidity, regardless of the mechanical activation. The mechanism of cocrystallization in the presence of moisture at deliquescent conditions usually consists of three stages: (1) moisture uptake, (2) dissolution of reactants and (3) cocrystal nucleation and growth (Figure 2) [21].
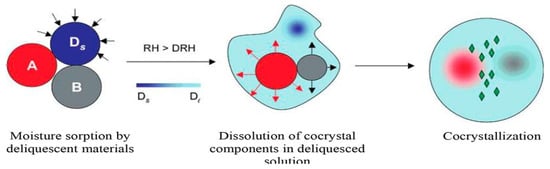
Figure 2.
Schematic representation of the moisture uptake process leading to deliquescence, reactant dissolution and cocrystal formation. A and B are cocrystal reactants, Ds is solid deliquescent additive, and Dl is the solution phase created by deliquescence at relative humidity greater than deliquescence relative humidity. (Reprinted with permission from [21]. Copyright 2007 American Chemical Society).
2.1.1. Contact Formation
The spontaneous formation of cocrystals via the mixing of pure API and coformer under a controlled atmospheric environment has already mentioned. In this method, no mechanical forces are applied during cocrystallization. However, in some cases, a brief grinding of the pure components—individually, before mixing—has been carried out. It has been observed that the cocrystallization rate in the case of premilled reactants was markedly faster than that of unmilled reactants. Moreover, higher cocrystallization rates have been observed for the same system at higher temperatures and relative humidity, regardless of the mechanical activation [22].
2.1.2. Solid State Grinding
Solid state grinding methods are widely used to generate cocrystal powder samples. Two formats are practiced: (1) neat (dry) grinding and (2) liquid-assisted grinding. Dry grinding is a cocrystallization method without a solvent. The solid materials that will result in the cocrystal are admixed in appropriate stoichiometric amounts, pressed and crushed together with a mortar and pestle, or a ball mill or vibrator mill.
The common grinding duration ranges from 30 to 60 min. With this method, numerous cocrystals can be prepared, and any failure is generally due to the use of inappropriate settings. Reducing the particle size increases the specific surface area of interaction between the materials for the development of intermolecular bonds. This offers the advantage of increased selectivity compared to cocrystallization through dissolution. It is simple, and allows quick preparation of the desired cocrystal. It has also been used as a method of clarifying hydrogen bond preference. But issues with dry grinding can include failure to form a cocrystal, incomplete conversion to the cocrystal and crystalline defects with the possible generation of some amorphous content. Similar way incomplete conversion to the cocrystal, resulting in a mixture of cocrystals and excess starting material in the product, is not desirable because then requires the use of addition purification steps to yield a pure cocrystal product [23].
2.1.3. Liquid-Assisted Grinding
This is a modification of neat grinding method. It includes mixing the two components and adding a very small amount of solvent (for example a few tenths of an equivalent of solvent per mole of the component) during the grinding process resulting in a significantly higher kinetics of cocrystal formation. In this process, solvent act as a catalyst, either as media that facilitate molecular diffusion or as an important factor that forms multi components inclusion framework and has been used to enhance supramolecular selectivity in crystalline systems. The effect of the solvent can be described as catalytic, as its small amount is not part of the final product.
Its advantages lie in its increased performance, in the ability to control the production of polymorphs, and in the improved crystallinity of the product, while a large number of coformers are suitable for the cocrystallization. This method enhances the cocrystallization rate, as some cocrystals showed poor performance in cocrystal formation following neat grinding for a considerable amount of time. This method can be used to prepare high-purity cocrystals with a significant reduction in the preparation time. It also allows the synthesis of selective polymorphic forms of cocrystals. This allows interconversion between crystalline forms of polymorphic organic components, according to polarity of the solvent. Limitations of liquid-assisting grinding include the fact that it is a small-scale technique, requires high energy consumption, and has a low performance in terms of product purity [23].
2.1.4. Hot Melt Extrusion Technique
Hot-Melt Extrusion (HME) method is a method that combined cocrystal formation and drug-formulation process, exhibit a simpler way to manufacture a drug product. In the hot melt extrusion (HME) technique, the cocrystals area unit is prepared by heating the drug and coformers with intense intermixture, which improved the surface contacts without using solvent. The heat that used for HME method is set at a specific temperature, where only the matrix is softened/ melted. Cocrystal formation using HME method requires a catalyzing agent to improve cocrystal formation played by softened/melted matrix. Suitable matrices for HME method must have several qualities; (1) have low glass transition (Tg) temperature, lower than melting point of cocrystal to ensure a lower processing temperature, (2) have limited noncovalent interaction with drug or conformer, (3) exhibit a rapid solidification step. The disadvantage of this methodology is that each coformer and API are not compatible with the liquefied kind and cannot be used for unstable medicine [24].
2.2. Solution Based Methods
A large no. of methods exists for cocrystallization from solution, and each will be discussed in the following section. The major driving force for crystallization is supersaturation. With a cocrystal system, there are two concentrations to be consider: (1) target molecule and (2) coformer. The concentrations of both relative to the solubility of the cocrystal decided the extent of supersaturation for cocrystallization. A eutectic point will exist where at one fixed solution concentration, a mixture of cocrystal and the target molecule is the stable solid phase for the system; Similar way a second eutectic point exists for a mixture of the cocrystal and coformer. The eutectics represent solution minima, where the solvent content is at its lowest value, means solubility is at its highest value at this point. The cocrystal will only be stable if it is less soluble than the target molecule or coformer, means the concentrations lying between this eutectic points.
It has been suggested that it may be useful to consider polymorphic compounds, which exist in more than one crystalline form as co- crystallizing components. If a molecular compound exists in several polymorphic forms it has demonstrated a structural flexibility and is not locked into a single type of crystalline lattice or packing mode. Thus, the chance of bringing such a molecule into a different packing arrangement in coexistence with another molecule is increased.
Figure 3 shows the typical ternary phase diagrams, which describe the three-phase behavior of a multicomponent system: API, coformer, cocrystal and solvent. It is also able to predict the pathway of cocrystal formation.
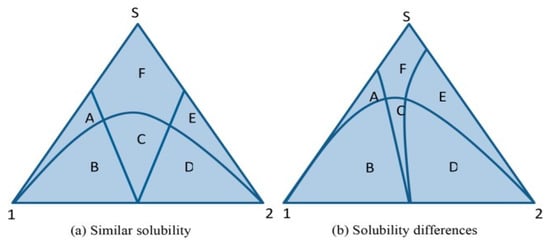
Figure 3.
Schematic representation of isothermal ternary phase diagram. (a) Similar solubilities between the active pharmaceutical ingredient (API) and coformer (1 and 2) in solvent S and (b) different solubilities of 1 and 2 in S. Region A, component 1 and solvent; B, component 1 + cocrystal; C, cocrystal; D, component 2 + cocrystal; E, component 2 and solvent; F, solution. (Modified from [25]. Copyright 2013 Cell Press).
2.2.1. Slurry Crystallization
Slurry crystallization is the method during which suspension is prepared by the addition of various solvents within the mixture of API and appropriate coformers. The solvent is decanted and then the solid material is dried under a flow of nitrogen for 5 min and characterized by using PXRD. This methodology is used for the preparation of cocrystals once the drug and coformer could be stable within the solvent [15].
2.2.2. Evaporative Cocrystallization
Evaporative cocrystallization is a common method of generating cocrystals, particularly used for generating single cocrystals which are suitable for diffraction studies. It is the most commonly used technique in the field of cocrystallization, in this technique stoichiometric amount of the conformer and the drug are dissolved in a common solvent. It is based on the principle that, when different molecules of complementary functional groups afford hydrogen bonds that are more favorable than each of the individual molecular components, the product formed is likely to be thermodynamically favored. The technique involves the nucleation and growth of a cocrystal from a solution of both coformers in a solvent, with supersaturation provided by removal of the solvent from the solution via evaporation. Individual cocrystals, or the bulk crystal sample, should be separated before the solution evaporates to dryness to ensure complete recovery of a clean crystals.
A slow rate of evaporation is usually preferred so as to ensure formation of a small number of larger crystals as opposed to a high number of smaller crystals. As crystal structure identification is an important step in the discovery of new cocrystal forms, evaporative cocrystallization is evident in the majority of cocrystal related research papers. It should be important to identify the crystal structure for defining whether the obtained crystal is a cocrystal, salt, hydrate, or any other polymorphic form of the API or coformer. Ideally, evaporative cocrystallization should be carried out from three solutions: 1:1 stoichiometric solution, a solution where the coformer is in excess and a solution where the target molecule is in excess [26].
2.2.3. Cooling Crystallization
It is less frequently applied method for the cocrystal formation. It is generally slow and time-consuming process as compared to other techniques, for example, darunavir-succinic acid cocrystal. In this, there is an improvement in solubility, dissolution, and micrometric properties than its individual drug darunavir. A designed seeded cooling crystallization was used to prepare cocrystals of carbamazepine: nicotinamide from ethanol in an effort to establish a scalable cocrystallization solution strategy. Solvent selection, identification of the thermodynamically stable Cocrystal operating range and de supersaturation kinetics were considered during the design of the process [25].
2.2.4. Anti-Solvent Method
Anti-solvent method also known as vapor diffusion is the methods in which antisolvent is used for the synthesis of high quality cocrystals. In this method, A solvent in which the compound is less soluble is often added to another solution, favoring the precipitation of the solids. During this process, supersaturation is generated by adding a second liquid to a solution of the drug-conformer to be crystallized, which is miscible with the solvent and in which the cocrystals are insoluble or sparingly soluble. The resulting suspension is filtered, and the collected solid can be characterized by XRPD. Disadvantages of this method are its lower performance as compared to grinding as well as the large volume of solvent required. In these studies, the construction of phase solubility diagrams was an integral part of the methodology for identifying the optimal concentration (e.g., ratio of solvent to antisolvent) for the formation of cocrystals. Generally, However, in many cases, a coformer solution is added to the drug organic solution to facilitate cocrystallization. This phenomenon is particularly used for the precipitation or recrystallization of the former and active pharmaceutical ingredients of the cocrystal. [27].
2.2.5. Crystallization by Reaction
Reaction crystallization methodology is used for the quick preparation of cocrystals at microscopic and macroscopic scale at a close temperature, during which the nucleation and cocrystallization depend upon the cocrystal elements and their solubility behaviour. The saturation of the lesser soluble part (drug) is formed in methanol and filtered, and therefore there is extra of the additional soluble part (coformer), in a quantity just below its solubility limit. The purpose of this study is to avoid use of any excess drug or coformer within the beginning solutions which could be confused as a cocrystal. Similarly, due to the solubility limits of the elements, the cocrystals which precipitate are unit pure. The concentrations area is monitored by HPLC (High pressure liquid chromatography) throughout the crystallization method to judge whether or not the solid ascertained gives the impression that it is like the cocrystals [28].
2.2.6. Ultrasound Aided Cocrystallization
Ultrasound aided cocrystallization or sonocrystallisation is another liquid assisted technology that has been employed for the synthesis of nano cocrystals. Sonocrystallization was first introduced by Childs et al. who used the sonic slurry method to synthesise cocrystals with a variety of drug—coformer pairs. In some experiments, sonication was repeated for 24 h on seeded or unseeded crystal slurries. In this this technique drug and coformers are dissolved in an appropriate vehicle (solvent). Cold water is pass throughout the sonication process to take care of the constant temperature of the sonicator and forestall fragmentation. The energy that is imparted to the sample during irradiation causes a rapid rise in temperature in a short period of time, which leads to melting of crystalline material, followed by material mixing and then rapid recrystallization upon cooling. One proposed condition for the coformer material which can be used for this method is that the coformer must be in sublimable condition in order to support a nucleation process through the vapor phase. Pure cocrystals were obtained by using this methodology [24].
2.2.7. Spray Flash Evaporation Process
Spray flash evaporation process technology was originally used in explosives in order to prepare semi-crystalline nano composites and is based on the flashing behavior of superheated liquid, which is subject to a rapid pressure drop. The process shows close interactions between various drug–coformer pairs and ultimately results into fast crystallization rates. The method involves dissolving the materials at a low boiling solvent (60 °C), over-pressurized at 40–60 bars following atomization into a chamber through a heated hollow cone nozzle. Due to the sudden fall in pressure drop, the superheated solution becomes thermodynamically unstable and the excess energy leads to converts into latent energy, producing cocrystallization of the compounds [24].
2.2.8. Supercritical Fluid Atomization Technique
Cocrystallization with supercritical solvent technique uses the solvent power of supercritical CO2 to suspend the API and the coformer as a slurry in liquid or supercritical CO2. Rapid expansion of supercritical solutions is a process in which a solution of the drug–coformer in supercritical CO2 is rapidly depressurized (10−5 s) to atmospheric conditions. Hence, the solvent power of the fluid drops dramatically produces high supersaturation of the solute in the depressurized supercritical CO2. The rapidly formed supersaturation leads to nucleation and crystallization, which subsequently forces the fine particles to precipitate. This technology uses non-toxic, highly volatile solvents without leaving any solvent residues to the final cocrystals. Some disadvantages of this method are the limited solubility of the drug–coformer pairs in supercritical CO2 and the low product yields [29].
2.2.9. Spray Drying Technique
Spray drying is a continuous single-step method of transformation of liquids (solutions, suspensions, slurries) to solid powders. In principle, spray-drying is the process of the transformation of a feed from the liquid stage to a dried particulate form by spraying the feed through a gaseous drying medium at elevated temperatures. Spray drying process that has been used for several pharmaceutical applications, such as micro and nano particles for pulmonary delivery, solid dispersions, viral vectors and pure drug particles. It is advantageous due to its continuous, highly controllable, and fast process. Spray drying has been widely used for formulating amorphous solid dispersions because of the fast solidification process. It is advantageous due to its continuous, highly controllable, and fast process.
Spray drying can also be used to produce cocrystals embedded in an excipient matrix with enhanced rheological properties. It was observed that a larger difference in Hansen Solubility Parameters between the cocrystal components and the excipient promotes cocrystal formation during spray drying in the presence of a carrier excipient. This leads to the cocrystal components (API and coformer) remaining phase separated from the excipient although interacting/cocrystallizing with each other, generating a cocrystal phase embedded in excipient matrix. For the drug–conformer incongruent solubility system, where pure cocrystal cannot be formed using the solvent evaporation method, cocrystallization using the spray-drying method can be used as an alternative method. Thus, the spray-drying method can provide a novel atmosphere for the preparation and scale-up of cocrystals [30].
2.3. Miscellaneous Cocrystal Preparation
2.3.1. Laser Irradiation
This method utilized high-power CO2 laser to irradiate powder blends of cocrystal formers and induce their recrystallization to a cocrystal structure. Titapiwatanakun et al. have used this method to produce caffeine cocrystals with oxalic acid and malonic acid. These authors have found that the cocrystal formers need to sublime to a considerable extent for the cocrystallization to take place, which indicated that the mechanism of the molecular rearrangement between drug and coformer molecules and the nucleation of the cocrystal is likely to take place in the vapor phase [31].
2.3.2. Resonant Acoustic Mixing
Resonant acoustic mixing has been used to mix the target molecule and coformer in the presence of a liquid to form a cocrystal in the absence of any grinding media. In this method, mechanical energy is transferred acoustically into a wetted powder mixture, initiating intimate mixing of the components. A range of carbamazepine cocrystals were successfully produced using a resonant acoustic mixer operating at 80–100 G and 60 Hz. The cocrystal products were isolated at a range of laboratory scales, 100 mg and 1.5 and 22 g, and the technology required to scale-up [32].
2.3.3. Freeze Drying
Freeze drying or lyophilization is another approach that has been used for the formation of pharmaceutical cocrystals. Recently, attempts have been made to adapt freeze drying for cocrystallization after it has already become an established process with several applications in biotechnology, pharmaceutical, diagnostics and food industries. Freeze drying is multistep operation by means of drying accomplished by freezing of a wet substance followed by ice sublimation directly to vapour by applying low partial pressure of water vapour. This method used as a processing technique to preserve a wide variety of products including food and pharmaceuticals. Recently, this method has also been demonstrated to be feasible for the preparation of new solid forms of cocrystal systems [33].
2.3.4. Electrospray Technology
Electro spraying is a process of simultaneous droplet generation and charging by means of an electric field. In this process, a solution containing the dissolved substances flows out from a capillary nozzle, which is maintained at high potential, through an electric field, which causes elongation of the solution droplets to form a jet. The solution jet is dried and the generated particles are collected on a charged powder collector [33].
2.3.5. Microfluidic and Jet Dispensing Approaches
Microfluidics is a versatile technology which allows assays to be conducted at very high throughput by running thousands of samples per second and controlling fluids in networks of micrometer-sized channels. According to this platform, the saturated solutions of parent compounds and coformers were dissolved in various solvents at very small quantities for a single chip through combinatorial mixing. By applying a two-phase screening process, caffeine was processed with a wide range of coformers and various solvents to identify combinations with the highest propensity for cocrystals. The parent compound (caffeine) was introduced into the chips vertically, while the coformers were introduced into the chips horizontally. The results proved that cocrystals screening using microfluidic chips is reliable and reproducible [34].
3. Evaluation of Cocrystals
3.1. Spectroscopic Analysis:
3.1.1. Fourier-Transform Infrared Spectroscopy:
It is widely used process for the prediction and determination of chemical conformation, intermolecular interactions, and communion study between API and coformers. Analysis of the API, coformers, and cocrystals has been performed by FTIR in the wavelength range of 400–4000 cm−1. This method is quick, nondestructive, prone to changes in molecular structure and can also detect a functional group.
3.1.2. Terahertz Time-Domain Spectroscopy:
This technique is similar to that of powder X-ray diffraction (PXRD) for the characterization and identification of cocrystals. It is useful for differentiating supramolecular structures, chiral, and racemic molecule present in a given sample. Example includes cocrystals of theophylline with different conformers.
3.1.3. Solid-State Nuclear Magnetic Resonance:
Solid state NMR is generally used for characterization and identification of various solid forms of pharmaceutical products including the cocrystals. This method identifies salts and cocrystals as well as evaluates the structure by detecting local conformation changes and hydrogen bonds by coupling the basic principle used in this method is the nuclei shift by irradiation that differs it from the excipients. By this quantitative and qualitative technique, we can determine the molar ratio of reaction mixture and type of hydrogen atom present in a given molecule.
3.2. Thermal Gravimetry Method:
This method is useful for determining the sample weight under the influence of temperature for a specific period of time. Differential scanning calorimetry (DSC): It is used for the determination of cocrystal formation, determined by the existence of exothermic crest followed by endothermic crest in the DSC spectra. The cocrystal formation is determined by the presence of crest (peaks) present in the compound. It is also useful for determining the melting point, polymorphic nature, glass temperature, heat of fusion, and exothermic or endothermic behavior of a compound or a molecule. Thermo gravimetric analysis gives exact drying temperature along the various reaction steps involved in the component. This method used for determination of hydrated or solvated forms of crystals, detection the volatile component as well as analyzing decomposition or sublimation from cocrystals. Prediction of crystal purity, solvates/hydrates forms of cocrystals, thermal stability and compatibility can be possible with Thermal gravimetry method.
3.3. Hansen Solubility Study:
Hansen solubility parameter is one of the important tools to predict the miscibility of a drug and coformer in crystal formation or with excipients/carriers. It can also predict compatibility of pharmaceutical materials and its study is also useful for the pre- formulation and formulation of tablets. The cohesion energy that is used to predict physiochemical properties such as melting point and solubility of a compound. Cocrystals are held together by a weak hydrogen bonding and are miscible at the molecular level.
In solubility study, different types of solvents are used such as water, buffer solutions of different pH, stimulated intestinal fluid, and gastric fluid. It is one of the important parameters of drug testing for drug development.
3.4. Dissolution Study:
It can be defined as “the quantity of drug substance that changes into a solution in a unit time in specific conditions of liquid/solid interface, solvent composition, and temperature.” In-vitro dissolution study of any solid drug is carried out to evaluate the dissolution efficacy of formulated drug. This study is performed on the dissolution apparatus in the suitable dissolution medium as per official compendia. The samples are collected at specified time interval are analyzed by HPLC or UV spectrophotometer. Solubility study Higuchi and Connors method is used to determine solubility of cocrystals. Solubility of cocrystals, pure API and physical mixture of API and coformer are determined in water and different medium as mentioned in official compendium.
3.5. Stability Study:
It is also one of the potent parameters for the evaluations of cocrystals [35] as it gives information about different climatic storage conditions and shelf life of the drug or drug products. There are various parameters that affect the stability of drug such as humidity, light, and temperature. Stability studies are performed at particular temperature and humidity conditions for predetermined time intervals which gives an idea about cocrystal product shelf life at various storage conditions.
4. Conclusions and Future Outlook
Cocrystals especially Pharmaceuticals, have become an important solid form in pharmaceutical space. It is evident from the number of research papers, review articles which are published in various journals as well as organization of conferences, symposiums and workshops in last decade. From the industrial point of view the number of patents filed throughout the world by various pharmaceutical industries and research groups are also increasing at a fast rate, since there is both regulatory and intellectual property relevance. Cocrystals are an excellent alternative for drug development in order to enhance solubility, bioavailability, stability and processability. However, several challenges remain, including coformer selection, physicochemical characterization and formulation. Careful drug conformer screening and formulation design can lead to successful cocrystals development. In this review, we discuss in detail a wide range of technologies applied for the experimental screening, synthesis and manufacturing of pharmaceutical cocrystals in order to overcome poor physical properties of APIs.
This review insight is provided on the proposed mechanisms of cocrystallization to be formed by different techniques. During early development, cocrystallization processes mainly focus on traditional methods, such as solvent evaporation, grinding and the slurry method. However, as time has gone by and the field has progressed, scientists in this field have developed newer methods which are increasingly simple to enable the cocrystallization processes to overcome their previous limitations. Novel methods that can be used for cocrystallization are hot melt extrusion, spray-drying, supercritical fluid technology, laser irradiation, freeze-drying, microfluidic and jet dispensing, etc. These methods successfully form various kinds of pharmaceutical cocrystals. However, every method still needs to be thoroughly investigated in order to better understand the clear cocrystallization mechanism for each method.
It is quite evident from the amount of interest shown by both academia and pharmaceutical industry that in near future pharmaceutical cocrystals will be one of the viable and important solid forms of pharmaceuticals for (i) Reformulation of existing drugs for improved performance. (ii) Life cycle management with recently approved drugs. (iii) Enabling novel development compounds; performance and purification. (iv) Green chemistry and synthesis with cocrystals as intermediates.
Acknowledgments
Writer is grateful to Noble Pharmacy College and Atmiya Institute of Pharmacy for their continuous support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Duarte, A.R.C.; Ferreira, A.S.D.; Barreiros, S.; Cabrita, E.; Reis, R.L.; Paiva, A. A comparison between pure active pharmaceutical ingredients and therapeutic deep eutectic solvents: Solubility and permeability studies. Eur. J. Pharm. Biopharm. 2017, 114, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.M.; Dressman, J.B. The Developability Classification System: Application of Biopharmaceutics Concepts to Formulation Development. J. Pharm. Sci. 2010, 99, 4940–4954. [Google Scholar] [CrossRef] [PubMed]
- Shan, N.; Zaworotko, M.J. The role of cocrystals in pharmaceutical science. Drug Discov. Today 2008, 13, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Bagde, S.A.; Upadhye, K.P.; Dixit, G.R.; Bakhle, S.S. Formulation and Evaluation of Cocrystals of Poorly Water Soluble Drug. Int. J. Pharm. Res. 2016, 7, 4988–4997. [Google Scholar]
- Brittain, H.G. Cocrystal systems of pharmaceutical interest: 2010. Cryst. Growth Des. 2011, 12, 1046–1054. [Google Scholar] [CrossRef]
- Jones, W.; Motherwell, W.S.; Trask, A.V. Pharmaceutical cocrystals: An emerging approach to physical property enhancement. MRS Bull. 2006, 31, 875–879. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Fasulo, M.E.; Desper, J. Cocrystal or salt: Does it really matter? Mol. Pharm. 2007, 4, 317–322. [Google Scholar] [CrossRef]
- Pindelska, E.; Sokal, A.; Kolodziejski, W. Pharmaceutical cocrystals, salts and polymorphs: Advanced characterization techniques. Adv. Drug Deliv. Rev. 2017, 1, 111–146. [Google Scholar] [CrossRef]
- Blagden, N.; de Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef]
- Aakery, C.B.; Salmon, D.J. Building Cocrystals with molecular sense and supramolecular sensibility. Cryst. Eng. Commun. 2005, 7, 439–448. [Google Scholar] [CrossRef]
- Billot, P.; Hosek, P.; Perrin, M.-A. Efficient Purification of an Active Pharmaceutical Ingredient via Cocrystallization: From Thermodynamics to Scale-Up. Org. Process Res. Dev. 2013, 17, 505–511. [Google Scholar] [CrossRef]
- Steed, J. The role of Cocrystals in Pharmaceutical Design. Trends Pharmacol. Sci. 2013, 34, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Blagden, N.; Berry, D.J.; Parkin, A.; Javed, H.; Ibrahim, A.; Gavan, P.T.; De Matos, L.L.; Seaton, C.C. Current directions in Cocrystal growth. New J. Chem. 2008, 32, 1659–1672. [Google Scholar] [CrossRef]
- Saxena, M.; Kuchekar, B.S. Cocrystal Formulation, Characterization, and Evaluation Study. In Proceedings of the International Conference on Advanced Nanomaterials & Emerging Engineering Technologies (ICANMEET-20J3), New Deihl, India, 24–26 July 2013; Volume 24, pp. 602–606. [Google Scholar]
- Douroumis, D.; Ross, S.A.; Nokhodchi, A. Advanced methodologies for cocrystal synthesis. Adv. Drug Deliv. Rev. 2017, 117, 178–195. [Google Scholar] [CrossRef]
- Mohana, M.; Muthiah, P.T.; McMillen, C.D. Supramolecular hydrogen-bonding patterns in 1:1 cocrystals of 5-fluorouracil with 4-methylbenzoic acid and 3-nitrobenzoic acid. Acta Cryst. 2017, 73, 259–263. [Google Scholar] [CrossRef]
- Oswald, I.D.H.; Motherwell, W.D.S.; Parsons, S. Formation of quinol Cocrystals with hydrogen-bond acceptors. Acta Cryst. 2005, 61, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Othman, M.F.; Jamburi, N.; Anuar, N.; Rahim, S.A.; Rohalim, N.H. Ibuprofen-amino acids Cocrystal screening via co-grinding methods. In MATEC Web of Conferences; EDP Sciences: Les Ulis, France, 2016; Volume 69. [Google Scholar]
- Setyawan, D.; Sari, R.; Yusuf, H.; Primaharinastiti, R. Preparation and Characterization of Artesunate-Nicotinamide Cocrystal by Solvent Evaporation and Slurry Method. Asian J. Pharm. Clin. Res. 2014, 7, 62–65. [Google Scholar]
- Chadha, R.; Bhalla, Y.; Vashisht, M.; Chadha, K. Cocrystallization in Nutraceuticals. In Recrystallization in Materials Processing; Intech Open: London, UK, 2015. [Google Scholar]
- Jayasankar, A.; Good, D.J.; Rodríguez-Hornedo, N. Mechanisms by which moisture generates Cocrystals. Mol. Pharm. 2007, 4, 360–372. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Nanda, A. Approaches to Design of Pharmaceutical Cocrystals: A Review. Mol. Cryst. Liquid Cryst. 2018, 667, 54–77. [Google Scholar] [CrossRef]
- Friscic, T.; Jones, W. Recent Advances in Understanding the Mechanism of Cocrystal Formation via Grinding. Cryst. Growth Des. 2009, 9, 1621–1637. [Google Scholar] [CrossRef]
- Li, S.; Yu, T.; Tian, Y.; Lagan, C.; Jones, D.S.; Andrews, G.P. Mechanochemical Synthesis of Pharmaceutical Cocrystal Suspensions via Hot Melt Extrusion: Enhancing Cocrystal Yield. Mol. Pharm. 2017, 15, 3741–3754. [Google Scholar] [CrossRef] [PubMed]
- Chadwick, K.; Davey, R.; Sadiq, G.; Cross, W.; Pritchard, R. The utility of a ternary phase diagram in the discovery of new cocrystal forms. Cryst Eng Comm 2009, 11, 412–414. [Google Scholar] [CrossRef]
- Desai, H.; Rao, L.; Amin, P. Carbamazepine Cocrystals by Solvent Evaporation Technique: Formulation and Characterization Studies. Am. J. Pharm. Res. 2018, 2, 4. [Google Scholar]
- Ober, C.A.; Gupta, R.B. Formation of Itraconazole–Succinic Acid Cocrystals by Gas Antisolvent Cocrystallization. AAPS PharmSciTech 2012, 13, 1396–1406. [Google Scholar] [CrossRef]
- Rodríguez-Hornedo, N.; Nehm, S.J.; Seefeldt, K.F.; Pagan-Torres, Y.; Falkiewicz, C.J. Reaction crystallization of pharmaceutical molecular complexes. Mol. Pharm. 2006, 3, 362–367. [Google Scholar] [CrossRef]
- Padrela, L.; Rodrigues, M.A.; Tiago, J.; Velaga, S.P.; Matos, H.A.; de Azevedo, E.G. Insight into the Mechanisms of Cocrystallization of Pharmaceuticals in Supercritical Solvents. Cryst. Growth Des. 2015, 15, 3175–3181. [Google Scholar] [CrossRef]
- Vehring, R. Pharmaceutical particle engineering via spray-drying. Pharm. Res. 2008, 25, 999–1022. [Google Scholar] [CrossRef]
- Titapiwatanakun, V.; Basit, A.W.; Gaisford, S. A New Method for Producing Pharmaceutical Cocrystals: Laser Irradiation of Power Blends. Cryst. Growth. 2016, 16, 3307–3312. [Google Scholar] [CrossRef]
- Chaudhary S, Nikam S, Khatri N, Wakde S, Co-Crystals: A review. J. Drug. Deliv. Ther. 2018, 8, 350–358.
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Athira, A.; Anu, S.; Thaifa, M. A Review on Pharmaceutical Cocrystals. Int. J. Pharm. Res. Scholars 2018, 7, 1–18. [Google Scholar] [CrossRef]
- Yadav, B.; Khusrsheed, A.; Sinh, R. Cocrystals: A Complete Review on Conventional and Novel Methods of its Formation and its Evaluation. Asian J. Pharm. Clin. Res. 2019, 12, 68–74. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).