Dose-Dependent Cytotoxicity of the Origanum vulgare and Carvacrol on Triple Negative Breast Cancer Cell Line †
Abstract
:1. Introduction
2. Methods
2.1. Chemicals
2.2. Plant Material
2.3. Preparation of Ov Infusion
2.4. Preparation of Crv Solution
2.5. Cell Lines and Cell Cultures
2.6. Cell Viability Assay and Cell Count
2.7. Cytotoxicity Test (MTT Test)
2.8. Statistical Analysis
3. Results and Discussion
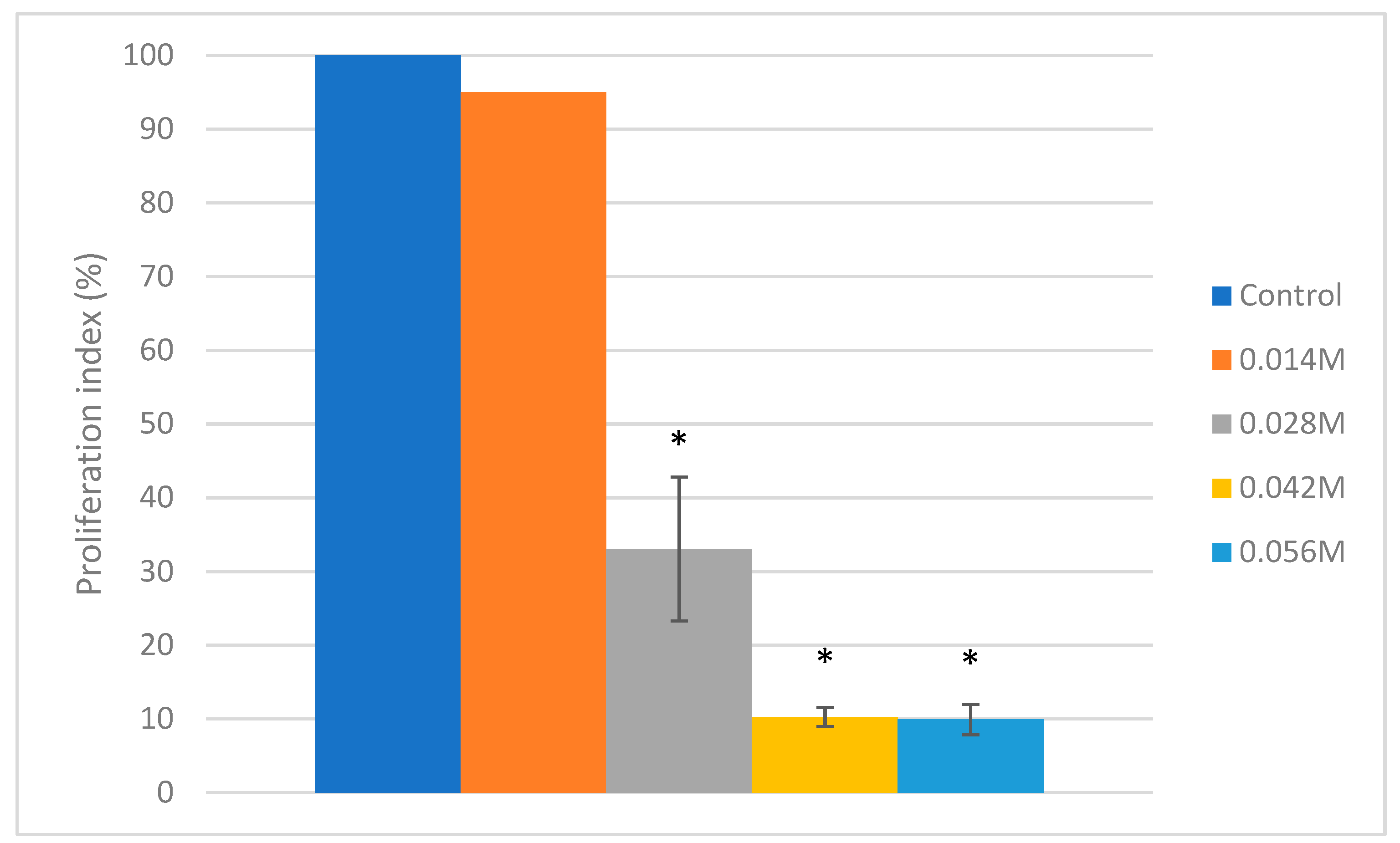
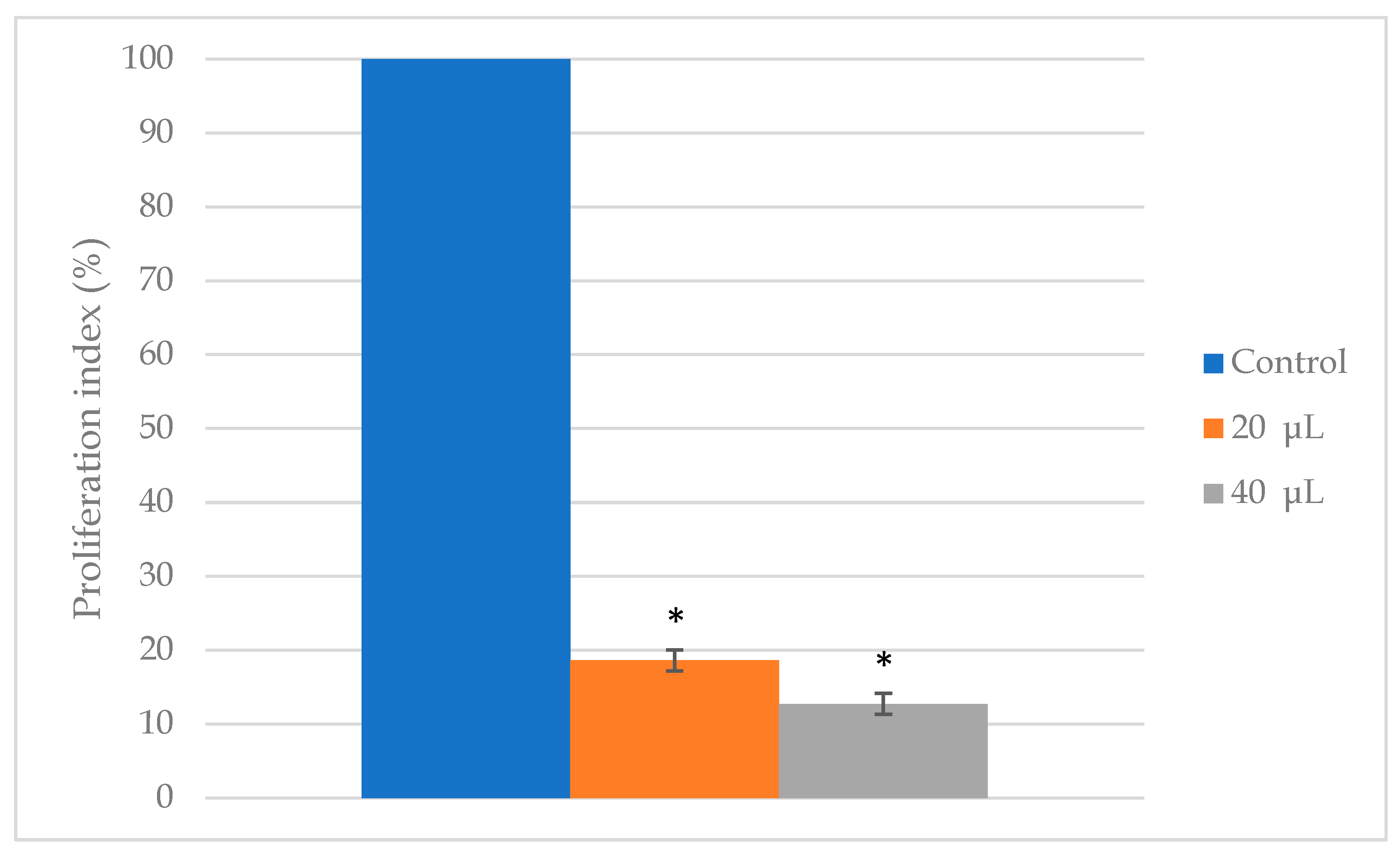
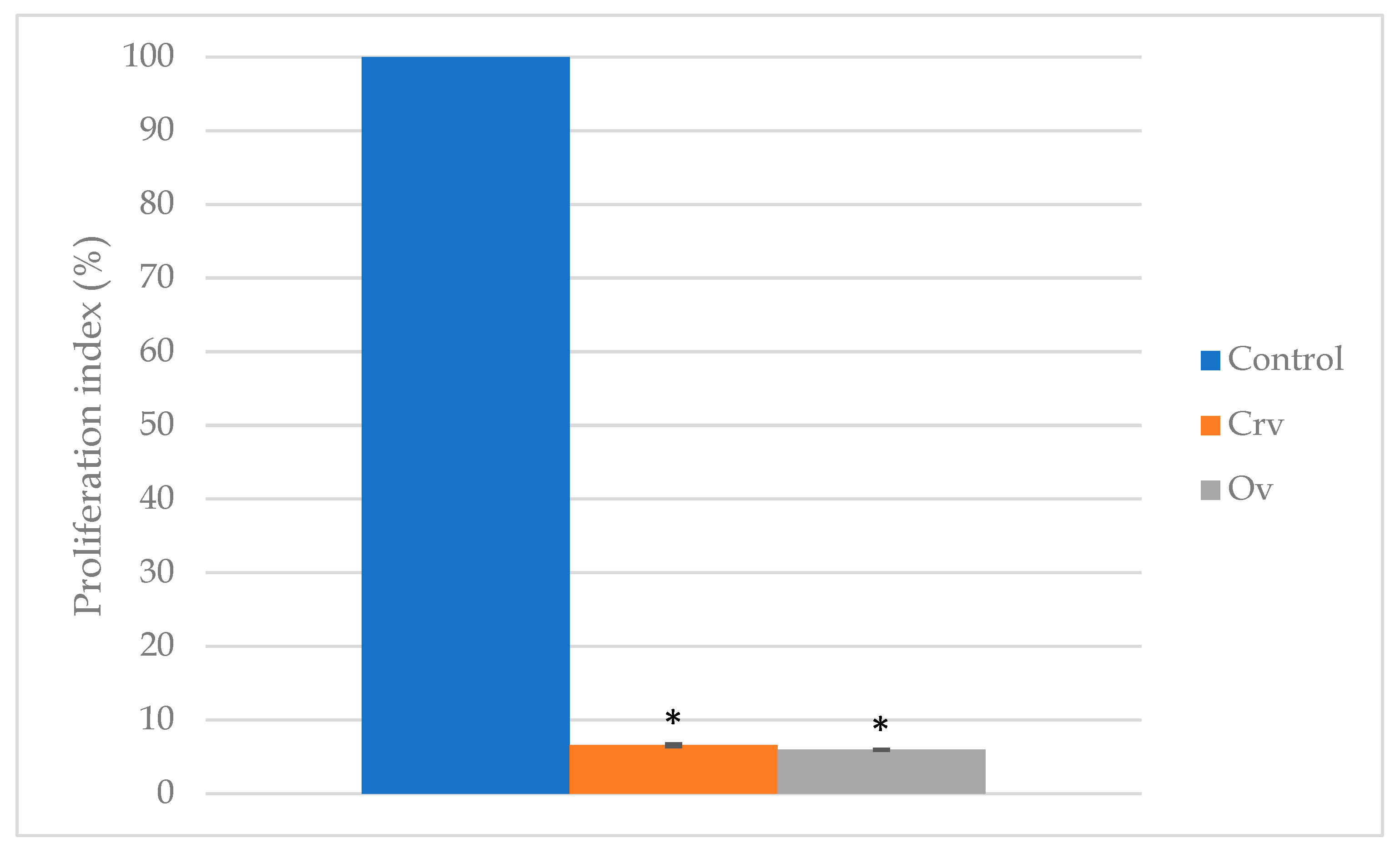
3.1. Cell Viability
3.2. Cytotoxicity
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- GLOBOCAN. All Cancers. 2018. Available online: http://gco.iarc.fr/today (accessed on 26 February 2020).
- GLOBOCAN. Breast. 2018. Available online: http://gco.iarc.fr/today (accessed on 26 February 2020).
- Perou, C.M.; Sørile, T.; Eisen, M.B.; Van De Rijn, M.; Jeffrey, S.S.; Ress, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef]
- OMS. OMS|Nuevas Directrices de la OMS para Fomentar el uso Adecuado de las Medicinas Tradicionales. WHO. 2013. Available online: https://www.who.int/mediacentre/news/releases/2004/pr44/es/ (accessed on 26 February 2020).
- Oniga, I.; Pușcaș, C.; Silaghi-Dumitrescu, R.; Olah, N.K.; Sevastre, B.; Marica, R.; Marcus, I.; Sevastre-Berghian, A.C.; Benedec, D.; Pop, C.E.; et al. Origanum vulgare ssp. vulgare: Chemical composition and biological studies. Molecules 2018, 23, 2077. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phyther. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- OMS. OMS|Cáncer. Available online: https://www.who.int/topics/cancer/es/ (accessed on 26 February 2020).
- CDC. ¿Qué es el cáncer de mama?|CDC. Available online: https://www.cdc.gov/spanish/cancer/breast/basic_info/what-is-breast-cancer.htm (accessed on 26 February 2020).
- ACS. ¿Qué es el Cáncer de Seno? Available online: https://www.cancer.org/es/cancer/cancer-de-seno/acerca/que-es-el-cancer-de-seno.html (accessed on 26 February 2020).
- Imigo, G.F.; Mansilla, S.E.; Delama, G.I.; Poblete, S.M.T.; Fonfach, Z.C. Clasificación molecular del cáncer de mama. Cuad. Cir. 2011, 25, 67–74. [Google Scholar] [CrossRef]
- CDC. Cáncer de Mama Triple Negativo|CDC. Available online: https://www.cdc.gov/spanish/cancer/breast/triple-negative.htm (accessed on 26 February 2020).
- Amin, A.R.M.R.; Kucuk, O.; Khuri, F.R.; Shin, D.M. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009, 27, 2712–2725. [Google Scholar] [CrossRef]
- Reynoso-Noverón, N.; Mohar, A. El cáncer en México: Propuestas para su control. Salud Publica Mex. 2014, 56, S27–S32. [Google Scholar] [CrossRef]
- Fitzmaurice, C.; Dicker, D.; Pain, A.; Hamavid, H.; Moradi-Lakeh, M.M.M. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef]
- Dias, D.A.; Urban, S.; Roessner, U. A Historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef]
- Cordell, G.; Colvard, M. Natural products and traditional medicine. J. Nat. Prod. 2012, 75, 514–525. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.-M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E. Discovery and resupply of pharmacologically active plant- derived natural products: A review. Biotechnol. Adv. 2015, 33, 1–18. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Khazir, J.; Mir, B.A.; Pilcher, L.; Riley, D.L. Role of plants in anticancer drug discovery. Phytochem Lett. 2014, 7, 173–181. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Maisanaba, S.; Puerto, M.; Pichardo, S.; Jos, A.; Moyano, R.; Cameán, A.M. A subchronic 90-day oral toxicity study of Origanum vulgare essential oil in rats. Food Chem. Toxicol. 2017, 101, 36–47. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Armentano, M.F.; Carmosino, M.; Bufo, S.A. Cytotoxic Activity of Origanum Vulgare L. on Hepatocellular Carcinoma cell Line HepG2 and Evaluation of its Biological Activity. Molecules 2017, 22, 1435. [Google Scholar] [CrossRef]
- Renukadevi, S.; Perumalsamy, H. Anti-proliferative activity of Origanum vulgare inhibited lipogenesis and induced mitochondrial mediated apoptosis in human stomach cancer cell lines. Biomed. Pharmacother. 2018, 108, 1835–1844. [Google Scholar] [CrossRef]
- Dragoeva, A.P.; Koleva, V.P.; Nanova, Z.D. Allelopathy of Cold Water Extracts from Origanum vulgare ssp. vulgare L. Sci. Res. 2014, 3, 144–150. [Google Scholar] [CrossRef]
- Brđanin, S.; Bogdanović, N.; Kolundžić, M.; Milenković, M. Antimicrobial activity of oregano (Origanum vulgare L.) and basil (Ocimum basilicum L.) Extracts. Adv. Technol. 2015, 4, 5–10. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, Y.; Wang, C.; Li, G.; Xu, J. Phenolic compounds from Origanum vulgare and their antioxidant and antiviral activities. Food Chem. 2014, 152, 300–306. [Google Scholar] [CrossRef]
- Gottumukkala Venkateswara Rao TMukhopadhyay TAnnamalai NRadhakrishnan, M.R.S. Chemical constituents and biological studies of Origanum vulgare Linn. Pharm. Res. 2011, 3, 143–146. [Google Scholar]
- Criollo-mendoza, M.S.; Id, G.V.; Heredia, J.B. Flavonoids and Phenolic Acids from Oregano: Occurrence, Biological Activity and Health Benefits. Plants 2017, 7, 1–23. [Google Scholar]
- Arigesavan, K.; Sudhandiran, G. Carvacrol exhibits anti-oxidant and anti-inflammatory effects against 1, 2-dimethyl hydrazine plus dextran sodium sulfate Induced inflammation associated carcinogenicity in the Colon of Fischer 344 rats. Biochem. Biophys. Res. Commun. 2015. [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Khan, F.; Khan, I.; Farooqui, A.; Ansari, I.A. Carvacrol Induces Reactive Carvacrol Induces Reactive Oxygen Species (ROS)-mediated Apoptosis Along with Cell Cycle Arrest at G0/G1 in Human Prostate Cancer Cells. Nutr. Cancer 2017, 69, 1075–1087. [Google Scholar] [CrossRef]
- Han, X.; Parker, T.L. Anti-inflammatory, tissue remodeling, immunomodulatory, and anticancer activities of oregano (Origanum vulgare) essential oil in a human skin disease model. Biochim. Open 2017, 4, 73–77. [Google Scholar] [CrossRef]
- Lim, W.; Ham, J. Carvacrol induces mitochondria-mediated apoptosis via disruption of calcium homeostasis in human choriocarcinoma cells. Cell Physiol. 2018, June, 1–13. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. In vitro and in vivo antitumor potential of carvacrol nanoemulsion against human lung adenocarcinoma A549 cells via mitochondrial mediated apoptosis. Sci. Rep. 2018, 8, 712–714. [Google Scholar] [CrossRef]
- Elbe, H.; Yigitturk, G.; Cavusoglu, T.; Baygar, T.; Ozgul Onal, M.; Ozturk, F. Comparison of ultrastructural changes and the anticarcinogenic effects of thymol and carvacrol on ovarian cancer cells: Which is more effective? Ultrastruct Pathol. 2020, 44, 193–202. [Google Scholar] [CrossRef]
- Arunasree, K.M. Anti-proliferative effects of carvacrol on a human metastatic BCcell line, MDA-MB 231. Phytomedicine 2010, 17, 581–588. [Google Scholar] [CrossRef]
- Vokou, D.; Kokkinit, S.; Bessiere, J. Geographic Variation of Greek Oregano (Origanum vulgare ssp. hirtum) Essential Oils. Biochem. Syst. Ecol. 1993, 21, 287–295. [Google Scholar] [CrossRef]
- Spyridopoulou, K.; Fitsiou, E.; Bouloukosta, E.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Oreopoulou, A.; Papavassilopoulou, E.; Pappa, A.; Chlichlia, K. Extraction, Chemical Composition, and Anticancer Potential of Origanum onites L. Essential Oil. Molecules 2019, 24, 2612. [Google Scholar] [CrossRef]
- Makrane, H.; El Messaoudi, M.; Melhaoui, A.; El Mzibri, M.; Benbacer, L.; Aziz, M. Cytotoxicity of the Aqueous Extract and Organic Fractions from Origanum majorana on Human Breast Cell Line MDA-MB-231 and Human Colon Cell Line HT-29. Adv. Pharm. Sci. 2018, 2018, 9. [Google Scholar] [CrossRef]
- Dhaheri YAl Attoub, S.; Arafat, K.; Abuqamar, S.; Viallet, J.; Saleh, A.; Agha, H.A.; Eid, A.; Iratni, R. Anti-Metastatic and Anti-Tumor Growth Effects of Origanum majorana on Highly Metastatic Human BCCells: Inhibition of NFκB Signaling and Reduction of Nitric Oxide Production. PLoS ONE 2013, 8, 1–17. [Google Scholar]
- Cittera, A.; Cazzola, R.; Cestaro, B.; Precliniche, S. Antioxidant Properties of oregano (ohganum vulgare) leaf extracts. J. Food Biochem. 2000, 24, 453–465. [Google Scholar]
- Dhaheri YAl Eid, A.; Abuqamar, S.; Attoub, S.; Khasawneh, M. Mitotic Arrest and Apoptosis in BCCells Induced by Origanummajorana Extract: Upregulation of TNF-a and Downregulation of Survivin and Mutant p53. PLoS ONE 2013, 8, 1–14. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojo-Ruvalcaba, B.E.; García-Cobián, T.A.; Pascoe-González, S.; Campos-Bayardo, T.I.; Guzmán-García, L.M.; Gil-Gálvez, M.C.; Escobar-Millán, Z.; Huerta-García, E.; García-Iglesias, T. Dose-Dependent Cytotoxicity of the Origanum vulgare and Carvacrol on Triple Negative Breast Cancer Cell Line. Proceedings 2020, 61, 6. https://doi.org/10.3390/IECN2020-07000
Rojo-Ruvalcaba BE, García-Cobián TA, Pascoe-González S, Campos-Bayardo TI, Guzmán-García LM, Gil-Gálvez MC, Escobar-Millán Z, Huerta-García E, García-Iglesias T. Dose-Dependent Cytotoxicity of the Origanum vulgare and Carvacrol on Triple Negative Breast Cancer Cell Line. Proceedings. 2020; 61(1):6. https://doi.org/10.3390/IECN2020-07000
Chicago/Turabian StyleRojo-Ruvalcaba, Brian Enrique, Teresa Arcelia García-Cobián, Sara Pascoe-González, Tannia Isabel Campos-Bayardo, Luz María Guzmán-García, María Cristina Gil-Gálvez, Zyanya Escobar-Millán, Eduardo Huerta-García, and Trinidad García-Iglesias. 2020. "Dose-Dependent Cytotoxicity of the Origanum vulgare and Carvacrol on Triple Negative Breast Cancer Cell Line" Proceedings 61, no. 1: 6. https://doi.org/10.3390/IECN2020-07000
APA StyleRojo-Ruvalcaba, B. E., García-Cobián, T. A., Pascoe-González, S., Campos-Bayardo, T. I., Guzmán-García, L. M., Gil-Gálvez, M. C., Escobar-Millán, Z., Huerta-García, E., & García-Iglesias, T. (2020). Dose-Dependent Cytotoxicity of the Origanum vulgare and Carvacrol on Triple Negative Breast Cancer Cell Line. Proceedings, 61(1), 6. https://doi.org/10.3390/IECN2020-07000



