“Green” Nanozymes: Synthesis, Characterization, and Application in Amperometric (Bio)sensors †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Enzymes
2.3. Synthesis of Hexacyanoferrates
2.4. Assay of Enzyme-Like Activities of the Synthesized HCFs
2.5. Characterization of the Synthesized HCFs
2.5.1. Optical Properties
2.5.2. Scanning Electron Microscopy (SEM)
2.5.3. FTIR Analysis
2.5.4. Particle Counter Analysis
2.5.5. Dynamic Light Scattering (DLS) Analysis and Zeta-Potential Measurements
2.6. Sensor Evaluation
2.6.1. Apparatus and Measurements
2.6.2. Immobilization of HCFs and the Enzyme onto Electrodes
3. Results
3.1. Green Synthesis of PBA
3.2. gHCFs as Artificial Peroxidases
3.2.1. Development and Characterization of gHCF-Modified Electrodes
3.2.2. Characterization of the Most Effective Electrodes
3.2.3. Application of gCuHCF as a PO Mimetic in Amperometric (Bio)sensors
3.3. Study of Structure, Morphology, and Size of the gCuHCF Composite
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABTS | 2,2′-azinobis (3-ethylbenzothiazoline-6-sulfonate) diammonium salt |
| chMHCF | Chemically synthesized HCF of a transitional metal M |
| CV | Cyclic voltammetry |
| DLS | Dynamic light scattering |
| DTT | Dithiothreitol |
| Fcb2 | Flavocytochrome b |
| FTIR | Fourier transform infrared spectroscopy |
| gMHCF | Green-synthesized hexacyanoferrate of a transitional metal M |
| gMHCF/GE | Green-synthesized hexacyanoferrate of a transitional metal M immobilized on GE |
| gNPs | Green-synthesized NPs |
| GE | Graphite electrode |
| GO | Glucose oxidase |
| gPBA | Green synthesized Prussian blue analogue |
| HCF | Hexacyanoferrate |
| hNFs | Organic-inorganic hybrid nanoflowers |
| Imax | Maximal current response on tested analyte at substrate saturation |
| KMapp | Apparent Michaelis–Menten constant |
| NaOAc | Sodium acetate buffer |
| NZs | Nanozymes |
| NPs | Nanoparticles |
| PAAG | Polyacrylamide gel |
| PB | Prussian blue |
| PBA | PB analogue |
| PO | Peroxidase |
| SAT | Standard addition test |
| SEM | Scanning electron microscope |
References
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2019, 48, 1004–1076. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Stasyuk, N.; Smutok, O.; Demkiv, O.; Prokopiv, T.; Gayda, G.; Nisnevitch, M.; Gonchar, M. Synthesis, Catalytic Properties and Application in Biosensorics of Nanozymes and Electronanocatalysts: A Review. Sensors 2020, 20, 4509–4550. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 5, 2190–2200. [Google Scholar] [CrossRef]
- Wang, P.; Wang, T.; Hong, J.; Yan, X.; Liang, M. Nanozymes: A New Disease Imaging Strategy. Front. Bioeng. Biotechnol. 2020, 8, 1–10. [Google Scholar] [CrossRef]
- Nayl, A.A.; Abd-Elhamid, A.I.; El-Moghazy, A.Y.; Hussin, M.; Abu-Saied, M.A.; El-Shanshory, A.A.; Soliman, H.M.A. The nanomaterials and recent progress in biosensing systems: A review. Trends Environ. Anal. Chem. 2020, 26, e00087. [Google Scholar] [CrossRef]
- Mahmudunnabi, R.; Farhana, F.Z.; Kashaninejad, N.; Firoz, S.H.; Shim, Y.B.; Shiddiky, M.J.A. Nanozyme-based electrochemical biosensors for disease biomarker detection. Analyst 2020, 145, 4398–4420. [Google Scholar] [CrossRef]
- Guari, Y.; Larionova, J. (Eds.) Prussian Blue-Type Nanoparticles and Nanocomposites: Synthesis, Devices, and Applications: Synthesis, Devices, and Applications; Pan Stanford Publishing Pte Ltd: Singapore, 2019; 314p, ISBN 978-981-4800-05-1. [Google Scholar]
- Ivanov, V.D. Four decades of electrochemical investigation of Prussian blue. Ionics 2020, 26, 531–547. [Google Scholar] [CrossRef]
- Ojwang, D.O. Prussian Blue Analogue Copper Hexacyanoferrate: Synthesis, Structure Characterization and Its Applications as Battery Electrode and CO2 Adsorbent. Ph.D. Thesis, Stockholm University, Stockholm, Sweden, 13 October 2017. Available online: http://www.diva-portal.org/smash/record.jsf?pid=diva2%3A1136799&dswid=8693 (accessed on 10 July 2020).
- Matos-Peralta, Y.; Antuch, M. Review—Prussian Blue and its analogs as appealing materials for electrochemical sensing and biosensing. J. Electrochem. Soc. 2020, 167, 037510. [Google Scholar] [CrossRef]
- Rauwel, P.; Rauwel, E. Towards the Extraction of Radioactive Cesium-137 from Water via Graphene/CNT and Nanostructured Prussian Blue Hybrid Nanocomposites: A Review. Nanomaterials 2019, 9, 682–703. [Google Scholar] [CrossRef]
- Jia, Q.; Li, Z.; Guo, C.; Huang, X.; Kang, M.; Song, Y.; He, L.; Zhou, N.; Wang, M.; Zhang, Z.; et al. PEGMA-modified bimetallic NiCo Prussian blue analogue doped with Tb(III) ions: Efficiently pH-responsive and controlled release system for anticancer drug. Chem. Eng. 2020, 389, 124468. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Li, F.; Han, X.; Chen, G. Prussian blue-coated lanthanide-doped core/shell/shell nanocrystals for NIR-II image-guided photothermal therapy. Nanoscale 2019, 11, 22079–22088. [Google Scholar] [CrossRef]
- He, L.; Li, Z.; Guo, C.; Hu, B.; Wang, M.; Zhang, Z.; Du, M. Bifunctional bioplatform based on NiCo Prussian blue analogue: Label-free impedimetric aptasensor for the early detection of carcino-embryonic antigen and living cancer cells. Sens. Actuators B Chem. 2019, 298, 126852. [Google Scholar] [CrossRef]
- Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Catalytically Synthesized Prussian Blue Nanoparticles Defeating Natural Enzyme Peroxidase. J. Am.Chem. Soc. 2018, 140, 11302–11307. [Google Scholar] [CrossRef]
- Tian, M.; Xie, W.; Zhang, T.; Liu, Y.; Lu, Z.; Li, C.M.; Liu, Y. Sensitive lateral flow immunochromatographic strip with Prussian blue nanoparticles mediated signal generation and cascade amplification. Sens. Actuators B Chem. 2020, 309, 127728. [Google Scholar] [CrossRef]
- Chen, W.; Gao, G.; Jin, Y.; Deng, C. A facile biosensor for Aβ40O based on fluorescence quenching of prussian blue nanoparticles. Talanta 2020, 216, 120390. [Google Scholar] [CrossRef]
- Nai, J.; Lou, X.W.D. Hollow Structures Based on Prussian Blue and Its Analogs for Electrochemical Energy Storage and Conversion. Adv. Mater. 2019, 31, 1706825. [Google Scholar] [CrossRef]
- Itaya, K.; Shoji, N.; Uchida, I. (Catalysis of the reduction of molecular oxygen to water at Prussian blue modified electrodes. J. Am. Chem. Soc. 1984, 106, 3423–3429. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Karyakina, E.E.; Gorton, L. Amperometric Biosensor for Glutamate Using Prussian Blue-Based “Artificial Peroxidase” as a Transducer for Hydrogen Peroxide. Anal. Chem. 2000, 72, 1720–1723. [Google Scholar] [CrossRef]
- Komkova, M.A.; Karyakin, A.A.; Andreev, E.A. Power output of Prussian Blue based (bio)sensors as a function of analyte concentration: Towards wake-up signaling systems. J. Electroanal. Chem. 2019, 847, 113263. [Google Scholar] [CrossRef]
- Komkova, M.A.; Andreev, E.A.; Ibragimova, O.A.; Karyakin, A.A. Prussian Blue based flow-through (bio)sensors in power generation mode: New horizons for electrochemical analyzers. Sens. Actuators B Chem. 2019, 292, 284–288. [Google Scholar] [CrossRef]
- Karyakin, A.A. Advances of Prussian blue and its analogues in (bio)sensors. Curr. Opin. Electrochem. 2017, 5, 92–98. [Google Scholar] [CrossRef]
- Jassal, V.; Shanker, U.; Kaith, B.S. Aegle marmelos mediated green synthesis of different nanostructured metal hexacyanoferrates: Activity against photodegradation of harmful organic dyes. Hindawi. Pub. Corp. 2016, 2016, 2715026. [Google Scholar] [CrossRef]
- Lee, I.; Kang, S.M.; Jang, S.C.; Lee, G.W.; Shim, H.E.; Rethinasabapathy, M.; Roh, C.; Huh, Y.S. One-pot gamma ray-induced green synthesis of a Prussian blue-laden polyvinylpyrrolidone/reduced graphene oxide aerogel for the removal of hazardous pollutants. J. Mater. Chem. A 2019, 7, 1737–1748. [Google Scholar] [CrossRef]
- Gour, A.; Jain, N.K. Advances in green synthesis of nanoparticles. Artif. Cells Nanomed. Biotechnol. 2019, 47, 844–851. [Google Scholar] [CrossRef]
- Gayda, G.Z.; Demkiv, O.M.; Stasyuk, N.Y.; Serkiz, R.Y.; Lootsik, M.D.; Errachid, A.; Gonchar, M.V.; Nisnevitch, M. Metallic nanoparticles obtained via “green” synthesis as a platform for biosensor construction. Appl. Sci. 2019, 9, 720–736. [Google Scholar] [CrossRef]
- Chinnadayyala, S.R.; Santhosh, M.; Singh, N.K.; Goswami, P. Alcohol oxidase protein mediated in-situ synthesized and stabilized gold nanoparticles for developing amperometric alcohol biosensor. Biosens. Bioelectron. 2015, 69, 155–161. [Google Scholar] [CrossRef]
- Vetchinkina, E.P.; Loshchinina, E.A.; Vodolazov, I.R.; Kursky, V.F.; Dykman, L.A.; Nikitina, V.E. Biosynthesis of nanoparticles of metals and metalloids by basidiomycetes. Preparation of gold nanoparticles by using purified fungal phenol oxidases. Appl. Microbiol. Biotechnol. 2017, 101, 1047–1062. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; Oliva González, C.M.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef]
- Jassal, V.; Shanker, U.; Kaith, B.S.; Shankar, S. Green synthesis of potassium zinc hexacyanoferrate nanocubes and their potential application in photocatalytic degradation of organic dyes. RSC Adv. 2015, 5, 26141–26149. [Google Scholar] [CrossRef]
- Gaida, G.Z.; Stel’mashchuk, S.Y.; Smutok, O.V.; Gonchar, M.V. A new method of visualization of the enzymatic activity of flavocytochrome b2 in electrophoretograms. Appl. Biochem. Microbiol. 2003, 39, 221–223. [Google Scholar] [CrossRef]
- Gonchar, M.; Smutok, O.; Os’mak, H. Flavocytochrome b2-Based Enzymatic Composition, Method and Kit for L-Lactate. Patent Application PCT/US2008/069637, Publ.WO/2009/009656. 2009. Available online: http://www.wipo.int/pctdb/en/wo.jsp?WO=2009009656 (accessed on 14 August 2020).
- Synenka, M.M.; Stasyuk, N.Y.; Semashko, T.V.; Gayda, G.Z.; Mikhailova, R.V.; Gonchar, M.V. Immobilization of oxidoreductases at/on gold and silver nanoparticles. Stud. Biol. 2014, 8, 5–16. [Google Scholar] [CrossRef]
- IR Spectrum Table & Chart. Available online: https://www.sigmaaldrich.com/technical-documents/articles/biology/ir-spectrum-table.html (accessed on 7 September 2020).
- Mink, J.; Stirling, A.; Ojwang, D.O.; Svensson, G.; Mihály, J.; Németh, C.; Drees, M.; Hajba, L. Vibrational properties and bonding analysis of copper hexacyanoferrate complexes in solid state. Appl. Spectros. Rev. 2018, 54, 369–424. [Google Scholar] [CrossRef]
- Stasyuk, N.; Gayda, G.; Zakalskiy, A.; Zakalska, O.; Serkiz, R.; Gonchar, M. Amperometric biosensors based on oxidases and PtRu nanoparticles as artificial peroxidase. Food Chem. 2019, 285, 213–220. [Google Scholar] [CrossRef]
- Gayda, G.; Klepach, H.; Semashko, T.; Gonchar, M. Nanoparticles of noble metals for enzymatic sensors: An amperometric glucose biosensor for wine analysis. Sens. Lett. 2017, 15, 647–654. [Google Scholar] [CrossRef]
- Karyakin, A.A.; Gitelmacher, O.V.; Karyakina, E.E. Prussian Blue-Based First-Generation Biosensor. A Sensitive Amperometric Electrode for Glucose. Anal. Chem. 1995, 67, 2419–2423. [Google Scholar] [CrossRef]
- Keihan, A.H.; Karimi, R.R.; Sajjadi, S. Wide dynamic range and ultrasensitive detection of hydrogen peroxide based on beneficial role of gold nanoparticles on the electrochemical properties of prussian blue. J. Electroanal. Chem. 2020, 862, 114001. [Google Scholar] [CrossRef]
- Vokhmyanina, D.V.; Andreeva, K.D.; Komkova, M.A.; Karyakina, E.E.; Karyakin, A.A. Artificial peroxidase” nanozyme—Enzyme based lactate biosensor. Talanta 2020, 208, 120393. [Google Scholar] [CrossRef]
- Huang, J.; Lu, S.; Fang, X.; Yang, Z.; Liu, X.; Li, S.; Feng, X. Optimized deposition time boosts the performance of Prussian blue modified nanoporous gold electrodes for hydrogen peroxide monitoring. Nanotechnology 2020, 31, 045501. [Google Scholar] [CrossRef]
- Clausmeyer, J.; Actis, P.; Córdoba, A.L.; Korchev, Y.; Schuhmann, W. Nanosensors for the detection of hydrogen peroxide. Electrochem. Commun. 2014, 40, 28–30. [Google Scholar] [CrossRef]
- Valiūnienė, A.; Virbickas, P.; Rekertaitė, A.; Ramanavičius, A. Amperometric Glucose Biosensor Based on Titanium Electrode Modified with Prussian Blue Layer and Immobilized Glucose Oxidase. J. Electrochem. Soc. 2017, 164, B781–B784. [Google Scholar] [CrossRef]
- Virbickas, P.; Valiūnienė, A.; Kavaliauskaitė, G.; Ramanavicius, A. Prussian White-Based Optical Glucose Biosensor. J. Electrochem. Soc. 2019, 166, B927–B932. [Google Scholar] [CrossRef]
- Chen, J.; Yu, Q.; Fu, W.; Chen, X.; Quan Zhang, Q.; Dong, S.; Chen, H.; Zhang, S. A Highly Sensitive Amperometric Glutamate Oxidase Microbiosensor Based on a Reduced Graphene Oxide/Prussian Blue Nanocube/Gold Nanoparticle Composite Film-Modified Pt Electrode. Sensors 2020, 20, 2924–2938. [Google Scholar] [CrossRef]
- Puganova, E.A.; Karyakin, A.A. New materials based on nanostructured Prussian blue for development of hydrogen peroxide sensors. Sens. Actuators B 2005, 109, 167–170. [Google Scholar] [CrossRef]
- Niu, Q.; Bao, C.; Cao, X.; Liu, C.; Wang, H.; Lu, W. Ni–Fe PBA hollow nanocubes as efficient electrode materials for highly sensitive detection of guanine and hydrogen peroxide in human whole saliva. Biosens. Bioelectron. 2019, 141, 111445. [Google Scholar] [CrossRef]
- Pang, H.; Zhang, Y.; Cheng, T.; Lai, W.Y.; Huang, W. Uniform manganese hexacyanoferrate hydrate nanocubes featuring superior performance for low-cost supercapacitors and nonenzymatic electrochemical sensors. Nanoscale 2015, 7, 16012–16019. [Google Scholar] [CrossRef]
- Sitnikova, N.A.; Borisova, A.V.; Komkova, M.A.; Karyakin, A.A. Superstable advanced hydrogen peroxide transducer based on transition metal hexacyanoferrates. Anal. Chem. 2011, 83, 2359–2363. [Google Scholar] [CrossRef]
- Pandey, P.C.; Panday, D.; Pandey, A.K. Polyethylenimine mediated synthesis of copper-iron and nickel-iron hexacyanoferrate nanoparticles and their electroanalytical applications. J. Electroanal. Chem. 2016, 780, 90–102. [Google Scholar] [CrossRef]
- Komkova, M.A.; Pasquarelli, A.; Andreev, E.A.; Galushin, A.A.; Karyakin, A.A. Prussian Blue modified boron-doped diamond interfaces for advanced H2O2 electrochemical sensors. Electrochim. Acta 2020, 339, 135924. [Google Scholar] [CrossRef]
- Ge, J.; Lei, J.; Zare, R.N. Protein–inorganic hybrid nanoflowers. Nat. Nanotechnol. 2012, 7, 428–432. [Google Scholar] [CrossRef]
- Zhu, J.; Wen, M.; Wen, W.; Du, D.; Zhang, X.; Wang, S.; Lin, Y. Recent progress in biosensors based on organic-inorganic hybrid nanoflowers. Biosens. Bioelectron. 2018, 120, 175–187. [Google Scholar] [CrossRef]
- Cui, J.; Jia, S. Organic–inorganic hybrid nanoflowers: A novel host platform for immobilizing biomolecules. Review. Coord. Chem. Rev. 2017, 352, 249–263. [Google Scholar] [CrossRef]
- Dong, W.; Chen, G.; Hu, X.; Zhang, X.; Shi, W.; Fu, Z. Molybdenum disulfides nanoflowers anchoring iron-based metal organic framework: A synergetic catalyst with superior peroxidase-mimicking activity for biosensing. Sens. Actuators. B. Chem. 2020, 305, 127530. [Google Scholar] [CrossRef]
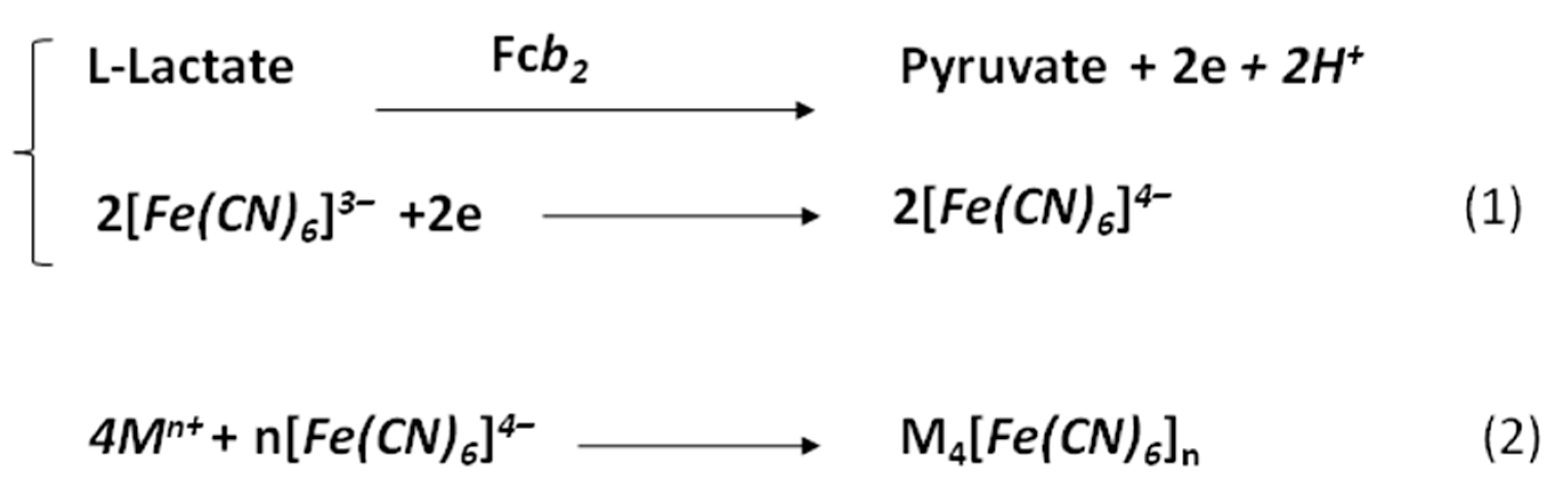
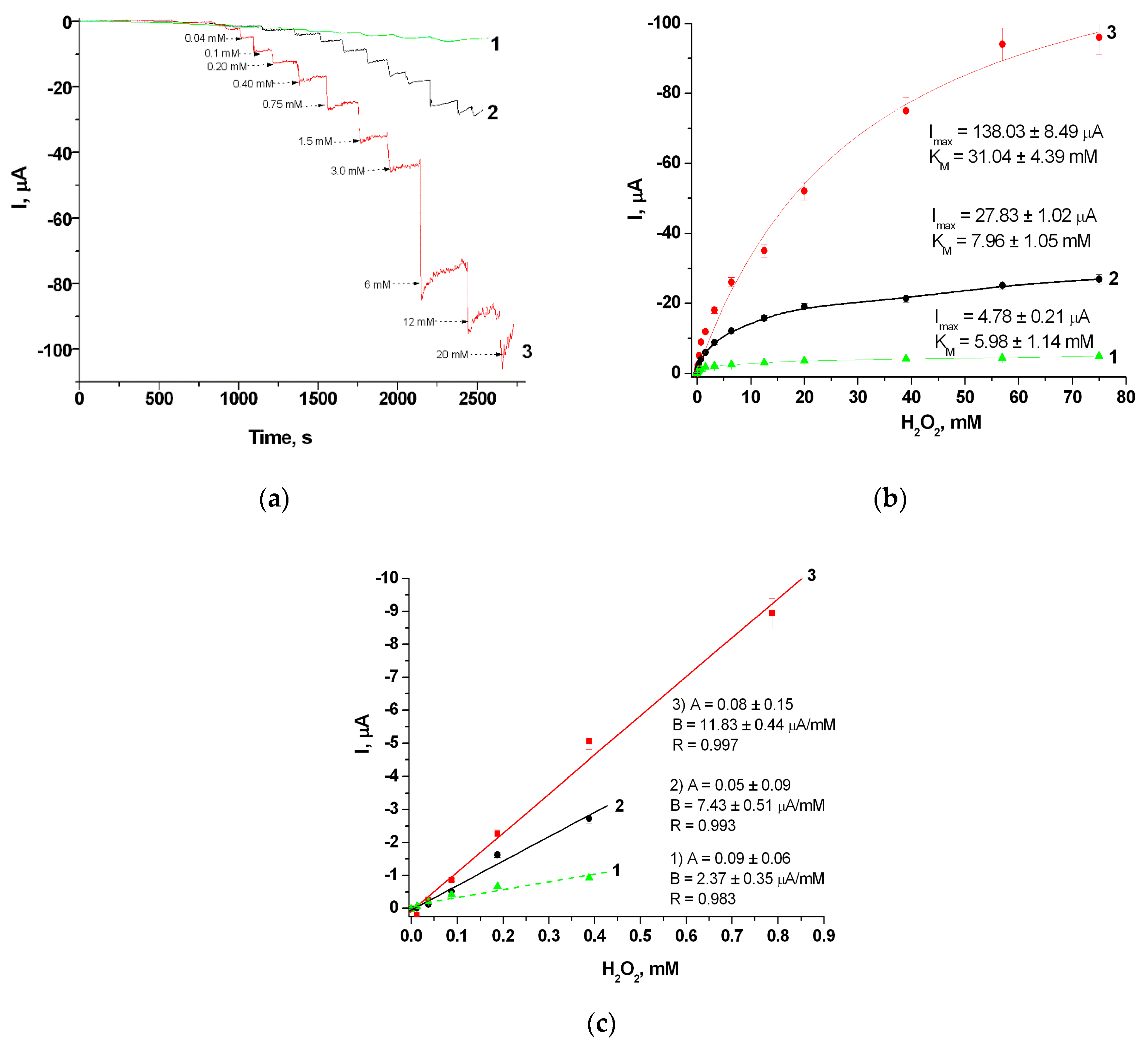
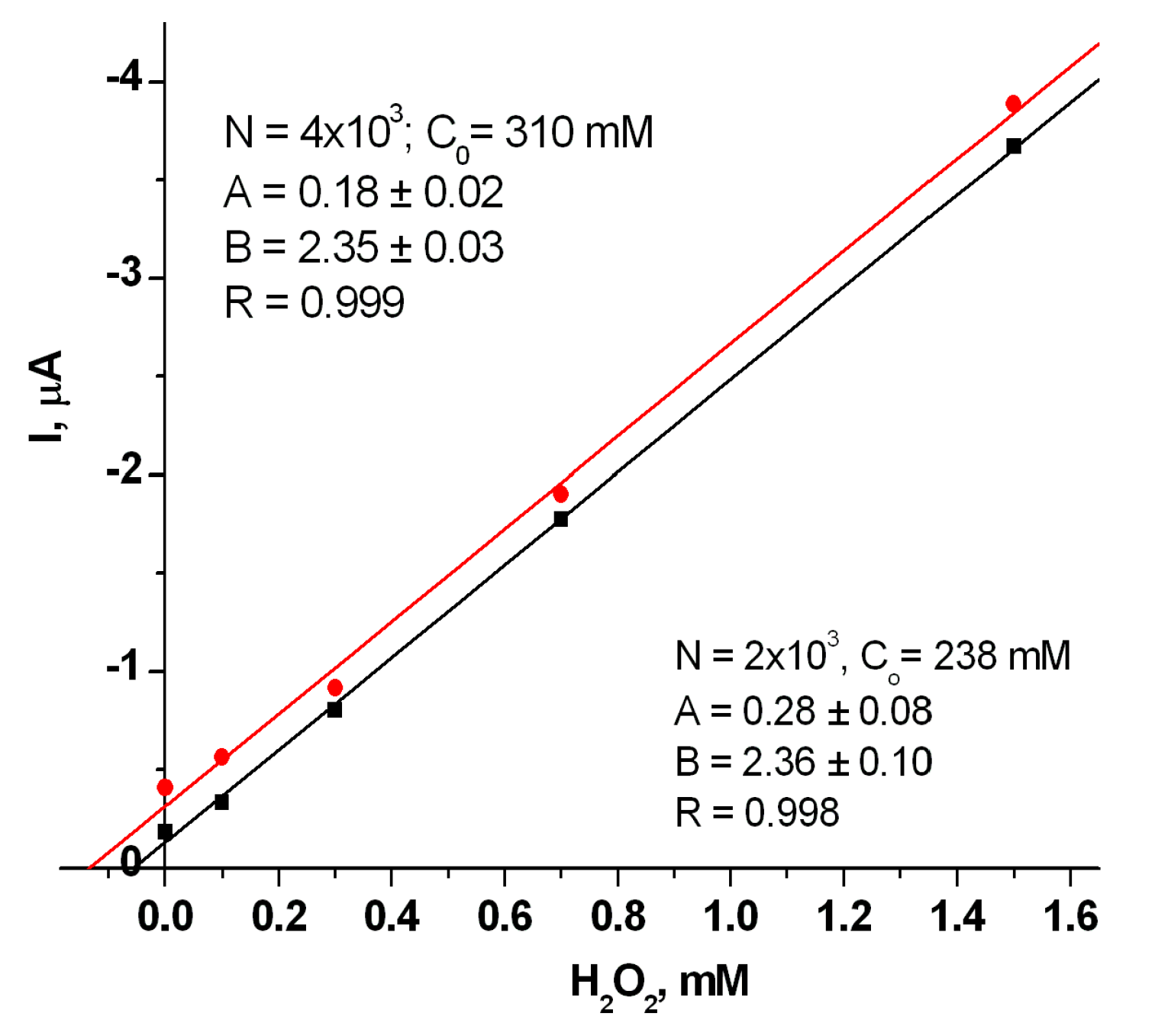
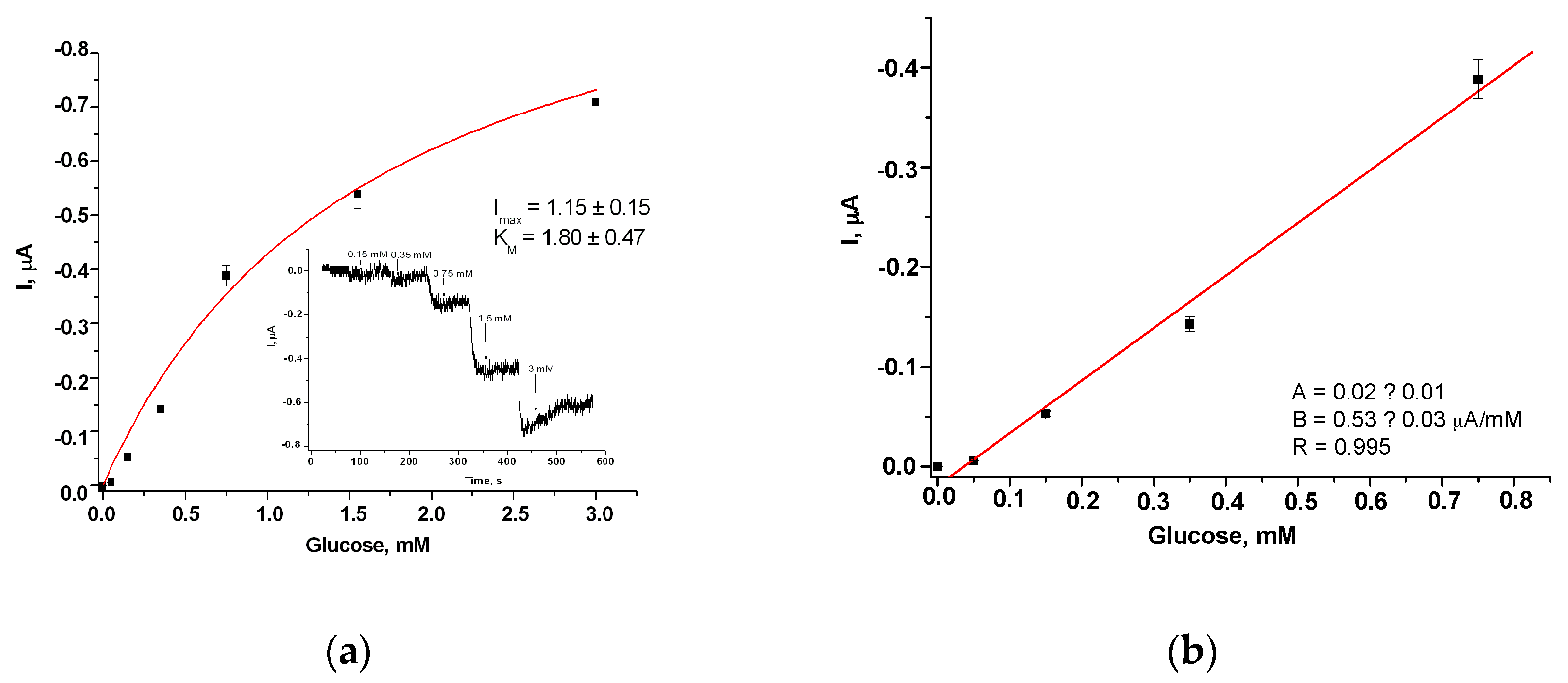
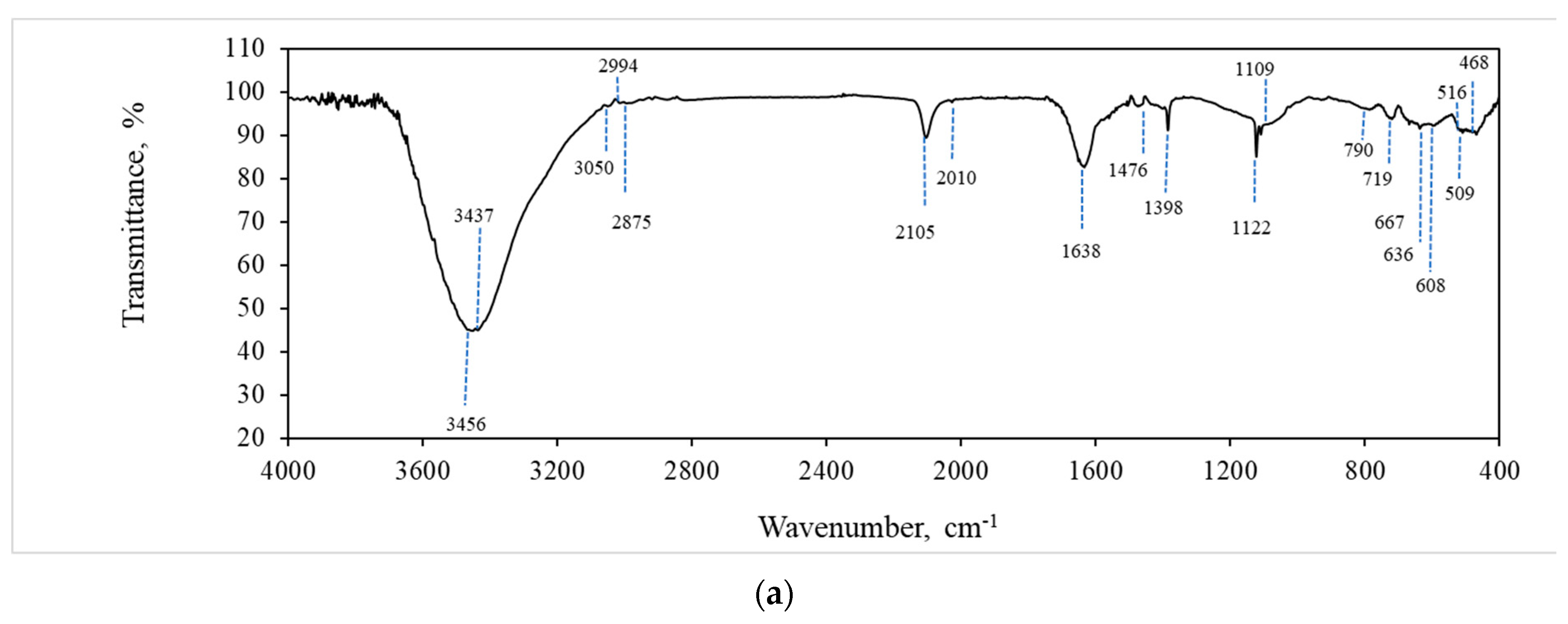
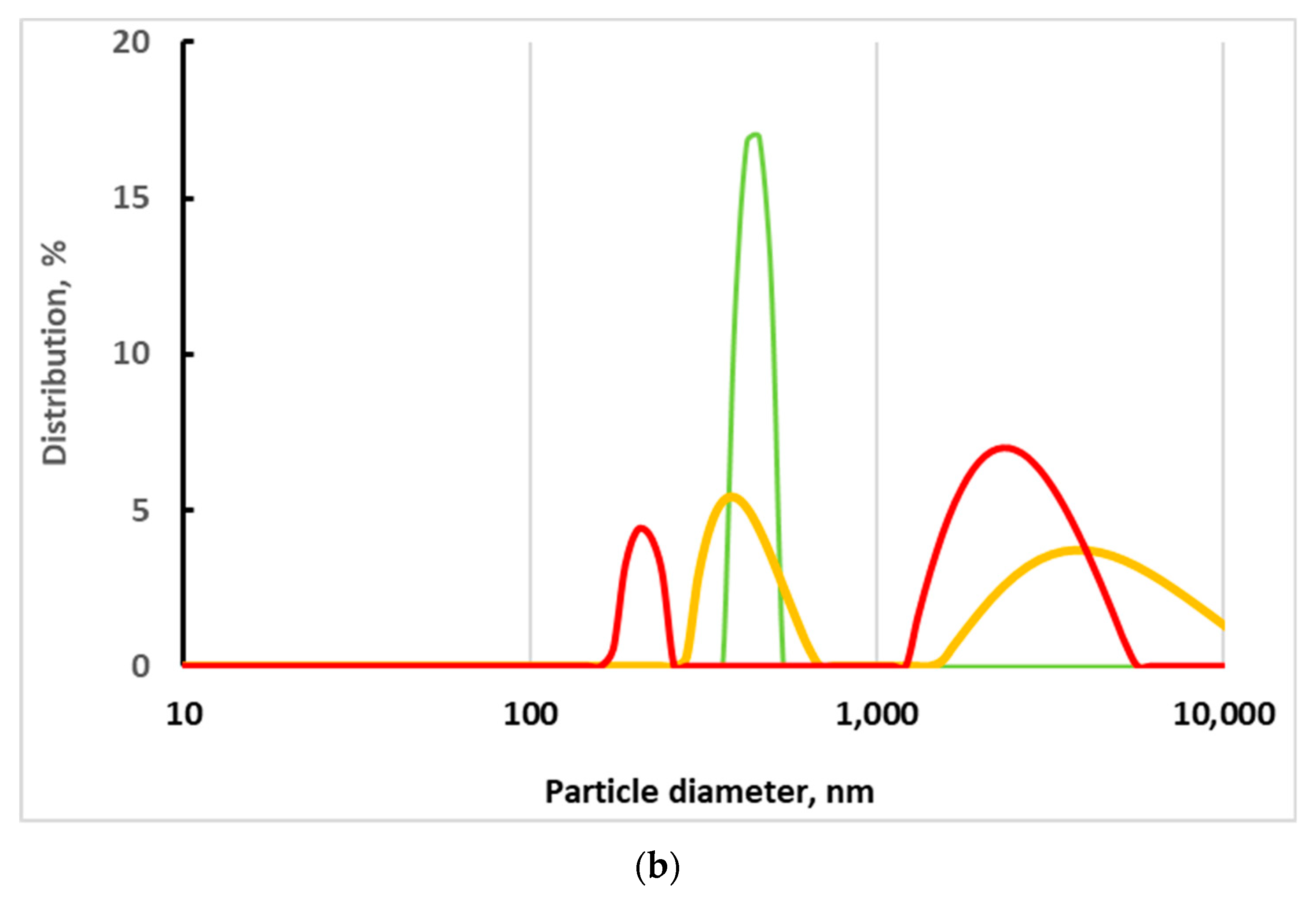
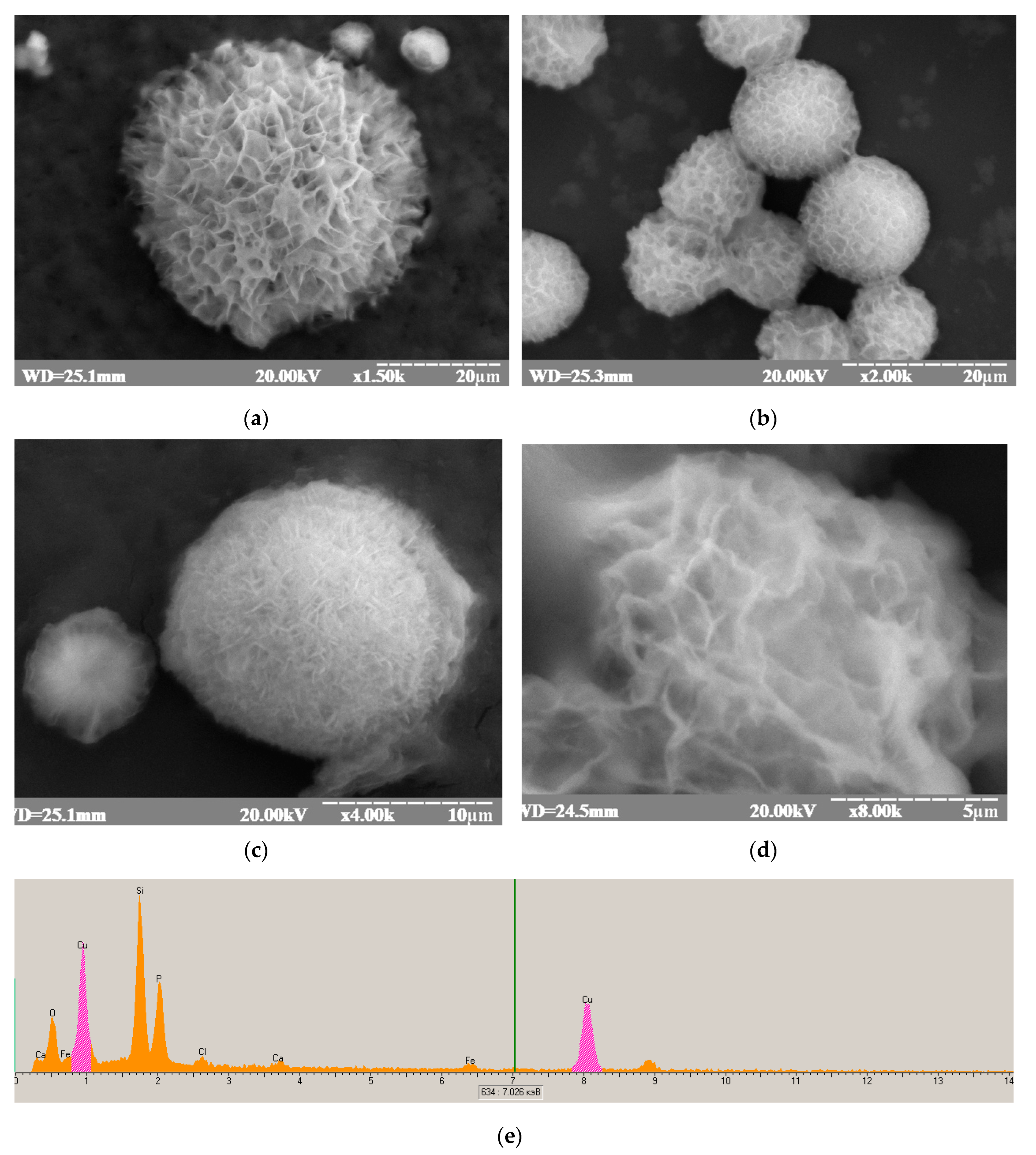
| Sensitive Film | KMapp, mM | ⃰ Imax, µA | Linear Range, up to, mM | Sensitivity, A M−1m−2 |
|---|---|---|---|---|
| gCuHCF | 31.0 ± 4.4 | 138.0 ± 8.5 | 0.8 | 1620 |
| gPB | 8.0 ± 1.1 | 27.8 ± 1.0 | 0.4 | 1090 |
| PO | 4.9 ± 1.1 | 5.0 ± 0.2 | 0.4 | 352 |
| chCuHCF | 20.0 ± 3.5 | 6.5 ± 0.4 | 0.8 | 110 |
| gMnHCF | 92.3 ± 15.2 | 21.1 ± 2.1 | 0.8 | 98 |
| gCoHCF | 26.1 ± 2.9 | 5.5 ± 0.2 | 3.5 | 29 |
| gZnHCF | 25.5 ± 2.2 | 4.0 ± 0.2 | 6.5 | 22 |
| gNdHCF | 21.3 ± 1.7 | 3.1 ± 0.1 | 6.5 | 16 |
| gCdHCF | 40.0 ± 5.4 | 2. 6 ± 0.2 | 1.5 | 15 |
| Particle Concentration, mL−1 | Hydrodynamic Diameter of the Peak 1, nm | Hydrodynamic Diameter of the Peak 2, nm | Polydispersity Index, % | Mean Zeta Potential, mV |
|---|---|---|---|---|
| 1.3 × 108 | 445 | * ND | 33.2 | −20.9 |
| 6.6 × 107 | 419 | 4876 | 43.2 | ND |
| 3.3 × 107 | 212 | 2635 | 35.9 | ND |
| Electrode | PO Mimetic | Sensitivity, A M−1m−2 | LOD, μM | Linear Range, μM | Ref. |
|---|---|---|---|---|---|
| GCE 1 | PB | 2000 | [48] | ||
| GCE | Ni–Fe PBA-4 HNCs | 361 | 0.1–20,000 | [49] | |
| GCE | MnPBA | 1472 | 3–8610 | [50] | |
| GCE | Ni-PB | 3500 | 0.1–1000 | [51] | |
| Graphite paste | Ni–FePBA Cu–FePBA | 1130 2030 | 2 0.2 | 2–1000 0.5–1000 | [52] |
| DBD 2 DBD | PB Ni–FePBA | 2100 1500 | 0.5 | 0.5–1000 | [53] |
| GE 3 | gCuPBA | 1620 | 2 | 10–800 | Current paper |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gayda, G.Z.; Demkiv, O.M.; Gurianov, Y.; Serkiz, R.Y.; Gonchar, M.V.; Nisnevitch, M. “Green” Nanozymes: Synthesis, Characterization, and Application in Amperometric (Bio)sensors. Proceedings 2020, 60, 58. https://doi.org/10.3390/IECB2020-07072
Gayda GZ, Demkiv OM, Gurianov Y, Serkiz RY, Gonchar MV, Nisnevitch M. “Green” Nanozymes: Synthesis, Characterization, and Application in Amperometric (Bio)sensors. Proceedings. 2020; 60(1):58. https://doi.org/10.3390/IECB2020-07072
Chicago/Turabian StyleGayda, Galina Z., Olha M. Demkiv, Yanna Gurianov, Roman Ya. Serkiz, Mykhailo V. Gonchar, and Marina Nisnevitch. 2020. "“Green” Nanozymes: Synthesis, Characterization, and Application in Amperometric (Bio)sensors" Proceedings 60, no. 1: 58. https://doi.org/10.3390/IECB2020-07072
APA StyleGayda, G. Z., Demkiv, O. M., Gurianov, Y., Serkiz, R. Y., Gonchar, M. V., & Nisnevitch, M. (2020). “Green” Nanozymes: Synthesis, Characterization, and Application in Amperometric (Bio)sensors. Proceedings, 60(1), 58. https://doi.org/10.3390/IECB2020-07072





