Electrochemical Platforms for Solid-Phase Isothermal Amplification and Detection of Bacterial Genome †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Instrumentation
2.3. Sensing Surface Construction onto ITO
2.4. Sensing Surface Construction onto Gold
3. Results and Discussion
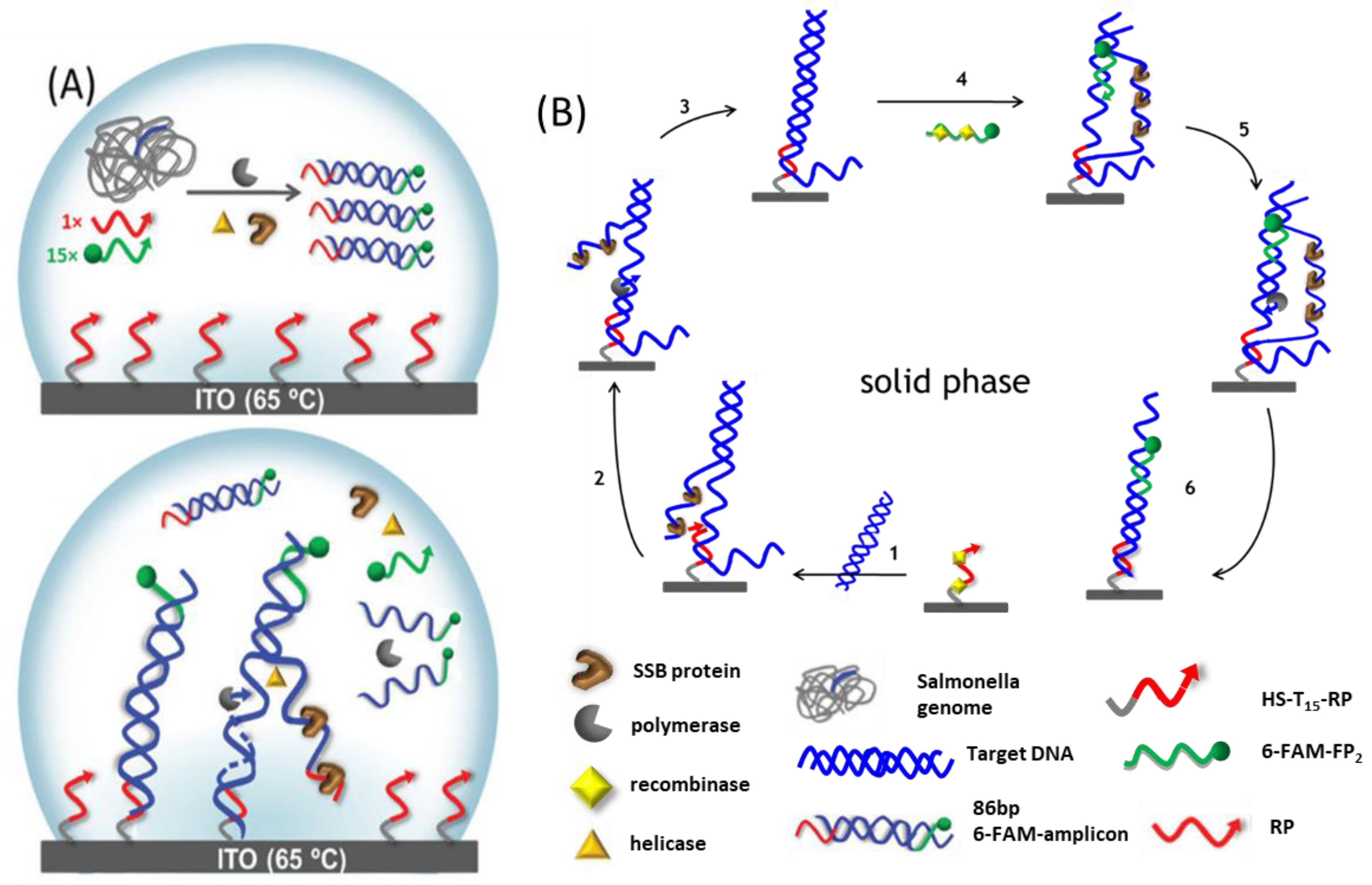
3.1. On-surface HDA
3.2. On-Surface RPA
3.3. Comparative Evaluation of the Performance of the Sensing Platforms
Funding
Conflicts of Interest
References
- Wu, G.; Zaman, M.H. Low-cost tools for diagnosing and monitoring HIV infection in low-resource settings. Bull. World Health Organ. 2012, 90, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Obande, G.A.; Singh, K.K.B. Current and future perspectives on isothermal nucleic acid amplification technologies for diagnosing infections. Infect. Drug Resist. 2020, 13, 455–483. [Google Scholar] [CrossRef] [PubMed]
- Barreda-García, S.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Solid-phase helicase dependent amplification and electrochemical detection of: Salmonella on highly stable oligonucleotide-modified ITO electrodes. Chem. Commun. 2017, 53, 9721–9724. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Salcedo, R.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J. On-gold recombinase polymerase primer elongation for electrochemical detection of bacterial genome: Mechanism insights and influencing factors. ChemElectroChem 2019, 6, 793–800. [Google Scholar] [CrossRef]
- Calvó, L.; Martínez-Planells, A.; Pardos-Bosch, J.; Garcia-Gil, L.J. A new Real-Time PCR assay for the specific detection of Salmonella spp. Targeting the bipA gene. Food Anal. Methods 2008, 1, 236–242. [Google Scholar] [CrossRef]
- Vincent, M.; Xu, Y.; Kong, H. Helicase-dependent isothermal DNA amplification. EMBO Rep. 2004, 5, 795–800. [Google Scholar] [CrossRef] [PubMed]
- Piepenburg, O.; Williams, C.H.; Stemple, D.L.; Armes, N.A. DNA detection using recombination proteins. PLoS Biol. 2006, 4, 1115–1121. [Google Scholar] [CrossRef] [PubMed]
- Miranda-Castro, R.; Sánchez-Salcedo, R.; Suárez-Álvarez, B.; de-los-Santos-Álvarez, N.; Miranda-Ordieres, A.J.; Lobo-Castañón, M.J. Thioaromatic DNA monolayers for target-amplification-free electrochemical sensing of environmental pathogenic bacteria. Biosens. Bioelectron. 2017, 92, 162–170. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Salcedo, R.; Miranda-Castro, R.; de-los-Santos-Álvarez, N.; Lobo-Castañón, M.J. Electrochemical Platforms for Solid-Phase Isothermal Amplification and Detection of Bacterial Genome. Proceedings 2020, 60, 23. https://doi.org/10.3390/IECB2020-07025
Sánchez-Salcedo R, Miranda-Castro R, de-los-Santos-Álvarez N, Lobo-Castañón MJ. Electrochemical Platforms for Solid-Phase Isothermal Amplification and Detection of Bacterial Genome. Proceedings. 2020; 60(1):23. https://doi.org/10.3390/IECB2020-07025
Chicago/Turabian StyleSánchez-Salcedo, Raquel, Rebeca Miranda-Castro, Noemí de-los-Santos-Álvarez, and María Jesús Lobo-Castañón. 2020. "Electrochemical Platforms for Solid-Phase Isothermal Amplification and Detection of Bacterial Genome" Proceedings 60, no. 1: 23. https://doi.org/10.3390/IECB2020-07025
APA StyleSánchez-Salcedo, R., Miranda-Castro, R., de-los-Santos-Álvarez, N., & Lobo-Castañón, M. J. (2020). Electrochemical Platforms for Solid-Phase Isothermal Amplification and Detection of Bacterial Genome. Proceedings, 60(1), 23. https://doi.org/10.3390/IECB2020-07025







