1. Introduction
The characterization of organic aerosols is a major task in current atmospheric research, mainly due to their complex composition and the physicochemical processes involved [
1,
2]. In ambient particulate matter, about 20 to 50% (and up to 90% in tropical forested areas) is comprised of organic aerosols [
3]. Organic aerosols have important contributions to the earth’s climate, affect air quality, and negatively impact human health [
4,
5]. Hundreds of thousands of different organic compounds have been measured in the atmosphere until now [
6] and, special attention is given nowadays to secondary organic aerosols (SOA) with poorly understood formation mechanisms and chemical composition. Liquid Chromatography tandem Mass Spectrometry (LC/MS) is an essential technique for the characterization of polar, nonvolatile, and/or thermally labile molecules that are more difficult to be analyzed by gas chromatography even with derivatization [
7]. In LC/MS techniques with electrospray ionization (ESI), achieving optimal LC/MS conditions represents a considerable challenge [
8,
9]. However, even under such conditions important contributors to atmospheric SOA, such as organosulfates, terpenoic acids (e.g., terebic, pinic, and pinonic acids), di- or tricarboxylic acids, nitrophenols and their related compounds, contributing to brown carbon or acting as tracers for biomass burning, are measured by LC/MS techniques in several laboratories [
10].
In organic aerosol investigations, evaluation of the matrix effect is a critical factor in establishing a reliable method. Co-eluted matrix compounds can induce analyte signal suppression or enhancement, which can lead to analyte under- or over-quantification in the sample. Therefore, it is strongly suggested to evaluate matrix effects during the LC/MS method development to ensure an acceptable final method precision, accuracy, specificity, and sensitivity. The influence of the co-extracted aerosol samples matrix on analyte detection and quantification in LC/ESI-MS analysis was observed and described for several analytes [
11,
12,
13].
The matrix effects contributing to the quantification of several organic aerosol components by high-performance liquid chromatography electrospray ionization time-of-flight mass spectrometry (HPLC/ESI-ToF-MS) are highlighted in the present work. The proposed analysis method aims at a minimal sample preparation to reduce the degradation and loss processes of labile compounds and solvent consumption. Investigations have been undertaken for relevant biogenic SOA tracers (e.g., terebic acid from α-pinene photo-oxidation), biomass burning tracers (e.g., 4-nitrocatechol, 4-nitrophenol, 2,6-dinitrophenol, 2,6-dimethyl-4-nitrophenol, phthalic acid, vanillic acid) and surrogate compounds for the semi-quantitation of organosulfates (camphor-10-sulfonic acid, sodium octyl sulfate).
2. Materials and Methods
Organic solvents from Sigma-Aldrich and ultrapure water (18.2 MΩ·cm) supplied by a Milli-Q water purification system (Millipore, Bedford, MA, USA) were used in the preparative steps. Standards for the aerosol organic chemical constituents were purchased from Sigma Aldrich or from TCI. An ESI-L Low Concentration Tuning Mix (Agilent Technologies, Santa Clara, CA, USA) and API-TOF Reference Mass Solution Kit (Agilent Technologies, Santa Clara, CA, USA) were used for mass spectrometer calibration and m/z correction during data acquisition.
Instrumental configuration used in this study is comprised of a high-performance liquid chromatography system (Agilent 1260 Infinity HPLC, Agilent Technologies, Waldbronn, Germany) coupled through a dual orthogonal electrospray ionization source (ESI) inlet to a time-of-flight mass spectrometer (6224 TOF-MS, Agilent Technologies, CA, USA). Chromatographic separation was carried out on a Poroshell 120 EC-C18 column (4.6 × 50 mm, 2.7 µm) from Agilent Technology. Analyte separation was achieved under an optimized gradient elution mode with acetonitrile and 0.1% (v/v) acetic acid solution.
The ESI-ToF-MS instrument was operated in negative ionization mode, and spectra were collected in full scan mode with 100–1100 m/z range and 1.4 spectra s−1 scan rate. In negative mode acquisition, for m/z correction during signal acquisition, 112.9856 m/z (trifluoroacetic acid), and 1033.9881 m/z (adduct of trifluoroacetic acid and hexakis(2,2,3,3-tetrafluoropropoxy)phosphazene) reference ions were selected.
Ambient atmospheric aerosols were collected in November 2019, in the Iasi urban area, Romania, at a sampling point located at about 35 m above the ground level. Atmospheric aerosols were collected on quartz filters (47 mm diameter, Whatman, Maidstone, UK) with ambient aerosol mass loading as high as 3014.0 µg. Quartz filters were cut in eight equal sections further used for matrix effect evaluation. The sample extraction was made with the Heidolph Reax top vortex mixer (500–2500 rotations per minute), homemade, adapted for the simultaneous extraction of 16 samples.
3. Results and Discussion
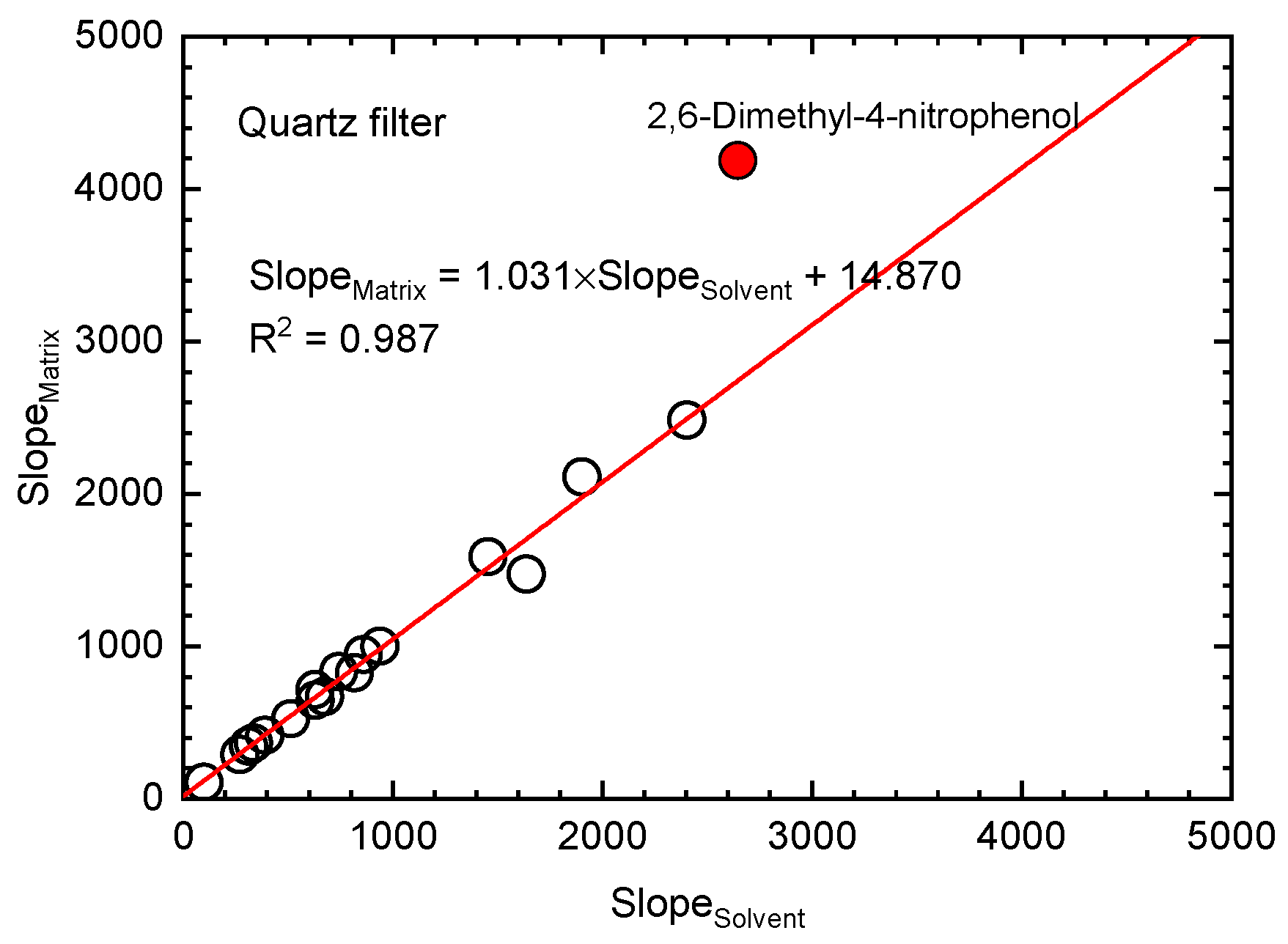
Quantitative information about the matrix effect intensity and its impact on mass accuracy measurements were gained by the post-extraction spiking method. Deprotonated ions ([M − H]
−)
m/
z values, which correspond to the primary signal, were used for ion extracted chromatograms and quantitative evaluation of the mass spectrometer response (area). Values of the matrix effects lower than 100% suggested the occurrence of a suppression effect, while values higher than 100% suggested the occurrence of an enhancement effect. Both signal suppression and enhancement effects were observed for different compounds. The matrix effect was evaluated for quartz filter samples, by calculating the ordinary least squares regression line slope ratios between the analyzed post-extraction spiked matrix-matched and target analytes using solvent-only in the same concentration range and the same solvent mixture. Using the graphic evaluation method, for most of the compounds, the matrix effect values were in the (89.9–113.8%) range, except for 2,6-dimethyl-4-nitrophenol (
Figure 1), which presents a high enhancement effect (158.2%). This suggests the presence of interference most probably due to other coeluted unidentified isomers from the sample matrix. Accuracy evaluation of analytes mass measurements for solvent and matrix spiked samples at different concentration levels (50–200 µg L
−1 and 400-1000 µg L
−1) (three replicates) shows higher mass measurement accuracy variability at lower concentrations (
Figure 2).
Statistically insignificant differences observed for analytes in spiked samples extracts and analytes in the solvent-only mixture indicate that the applied method is suitable for high accuracy mass measurements.
4. Conclusions
The developed analytical method for secondary organic aerosols tracers’ determination was tested for matrix effects on ESI response and mass accuracy measurements using atmospheric particulate matter samples collected on quartz filters. Satisfactory results were obtained for all the compounds except 2,6-dimethyl-4-nitrophenol. Additionally, measures are required to identify the interferences sources for these compounds. The accuracy of the molecular mass determination acquired in this study indicates that the presented method can contribute to new compounds identification from aerosol samples through molecular formula estimation.
Author Contributions
Conceptualization, C.A. (Cecilia Arsene) and R.I.O.; Methodology, data curation, and data analysis C.A. (Cornelia Amarandei), R.I.O. and C.A. (Cecilia Arsene); Formal analysis and investigation, C.A. (Cornelia Amarandei); Validation, C.A. (Cecilia Arsene) and R.I.O.; Writing—original draft preparation, C.A. (Cornelia Amarandei) and R.I.O.; Writing—review and editing, C.A. (Cecilia Arsene); Supervision, C.A. (Cecilia Arsene). All authors have read and agreed to the published version of the manuscript.
Acknowledgments
The authors acknowledge the financial support from European Union’s Horizon 2020 Research and Innovation Framework Program, through the EUROCHAMP-2020 Infrastructure Activity Grant (grant agreement No. 730997). The present work is related to the proposal PN-III-P4-ID-PCE-2020-1660 (SOS-ACHILE).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Srivastava, D.; Favez, O.; Bonnaire, N.; Lucarelli, F.; Haeffelin, M.; Perraudin, E.; Gros, V. Speciation of organic fractions does matter for aerosol source apportionment. Part 2: Intensive short-term campaign in the Paris area (France). Sci. Total Environ. 2018, 634, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Satish, R.V.; Rastogi, N. Characteristics and sources of fi ne organic aerosol over a big semi-arid urban city of western India using HR-ToF-AMS. Atmos. Environ. 2019, 208, 103–112. [Google Scholar] [CrossRef]
- Kanakidou, M.; Seinfeld, J.H.; Pandis, S.N.; Barnes, I.; Dentener, F.J.; Facchini, M.C.; Van Dingenen, R.; Ervens, B.; Nenes, A.; Nielsen, C.J.; et al. Organic aerosol and global climate modeling: A review. Atmos. Chem. Phys. 2005, 5, 1053–1123. [Google Scholar] [CrossRef]
- Heal, M.R.; Kumar, P.; Harrison, R.M. Particles, air quality, policy and health. Chem. Soc. Rev. 2012, 41, 6606–6630. [Google Scholar] [CrossRef] [PubMed]
- Weitekamp, C.A.; Stevens, T.; Stewart, M.J.; Bhave, P.; Gilmour, M.I. Health effects from freshly emitted versus oxidatively or photochemically aged air pollutants. Sci. Total Environ. 2020, 704, 135772. [Google Scholar] [CrossRef] [PubMed]
- Nozière, B.; Kalberer, M.; Claeys, M.; Allan, J.; D’Anna, B.; Decesari, S.; Finessi, E.; Glasius, M.; Grgić, I.; Hamilton, J.F.; et al. The molecular identification of organic compounds in the atmosphere: State of the art and challenges. Chem. Rev. 2015, 115, 3919–3983. [Google Scholar] [CrossRef] [PubMed]
- Olariu, R.I.; Vione, D.; Grinberg, N.; Arsene, C. Applications of liquid chromatographic techniques in the chemical characterization of atmospheric aerosols. J. Liq. Chromatogr. Relat. Technol. 2015, 38, 322–348. [Google Scholar] [CrossRef]
- Kostiainen, R.; Kauppila, T.J. Effect of eluent on the ionization process in liquid chromatography-mass spectrometry. J. Chromatogr. A 2009, 1216, 685–699. [Google Scholar] [CrossRef] [PubMed]
- Kruve, A.; Rebane, R.; Kipper, K.; Oldekop, M.L.; Evard, H.; Herodes, K.; Ravio, P.; Leito, I. Tutorial review on validation of liquid chromatography-mass spectrometry methods: Part II. Anal. Chim. Acta 2015, 870, 8–28. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.V.; Kerecman, D.E. Molecular characterization of atmospheric organic aerosol by mass spectrometry. Annu. Rev. Anal. Chem. 2019, 12, 247–274. [Google Scholar] [CrossRef] [PubMed]
- Warnke, J.; Bandur, R.; Hoffmann, T. Capillary-HPLC-ESI-MS/MS method for the determination of acidic products from the oxidation of monoterpenes in atmospheric aerosol samples. Anal. Bioanal. Chem. 2006, 385, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Kitanovski, Z.; Grgi, I.; Vermeylen, R.; Claeys, M.; Maenhaut, W. Liquid chromatography tandem mass spectrometry method for characterization of monoaromatic nitro-compounds in atmospheric particulate matter. J. Anal. Methods Chem. 2012, 1268, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Restivo, A.; Degano, I.; Ribechini, E.; Colombini, M.P. Development and optimisation of an HPLC-DAD-ESI-Q-ToF method for the determination of phenolic acids and derivatives. PLoS ONE 2014, 9, e88762. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).