Abstract
Water pollution is a critical environmental issue nowadays. One major problem is the pollution of freshwaters by pollutants of low concentrations (ng/L–μg/L), known as micropollutants. The most promising techniques for micropollutants degradation are Advanced Oxidation Processes (AOPs). Heterogeneous catalytic ozonation is among them, and recent studies have shown that it can be an efficient water treatment technique. The aim of this study is to evaluate the catalytic activity of five minerals (anatase, dolomite, kaolin, talc and zeolite) on the ozonation of small concentrations of p-CBA at pH 7 by batch mode experiments. p-CBA was employed as a model compound for evaluation of single and catalytic ozonation performance, because it cannot be efficiently removed by direct ozonation (kO3 < 0.15 M−1s−1), while it has high reactivity with hydroxyl radicals (k·OH = 5×109 M−1s−1). It was found that all applied solid materials can be characterized as catalysts, except kaolin, theuse of which presented almost the same performance with single ozonation. The best results were obtained by zeolite and dolomite (>99.4%) within 15 min reaction/oxidation time. These materials were neutrally (PZC = 6.8) and positively (PZC = 8.9) charged, respectively, during the oxidation process (pH 7), favoring the contact of micropollutant and ozone with the catalysts’ surface. On the other hand, the addition of anatase and talc in the ozonation system resulted in 97.5% and 98.5% p-CBA degradation, respectively, due to their slightly negative surface charge throughout the reaction. Conclusively, the experimental results indicated that the performance of heterogeneous catalytic ozonation is strongly depending on the surface charge of the solid materials (catalysts).
1. Introduction
The presence of micropollutants (μg/L–ng/L) in water significantly affects its quality. Ozonation is a long-studied method for water and wastewater treatment [1]. Ozone is an efficient oxidant, but it does not react with aldehydes and carboxylic acids, so it cannot achieve total mineralization. Furthermore, the oxidative reactions of ozone are slow and selective. For the obviation of these restrictions, Advance Oxidation Processes (AOPs) are applied. One of them is heterogeneous catalytic ozonation [2]. This process is based on ozone decomposition and the consequent production of hydroxyl radicals (•OH), which are more powerful oxidizing agents and react non-selectively [3]. In many studies, minerals have been used as potential catalysts in heterogeneous catalytic ozonation, due to their low cost, low toxicity and common availability[4,5].
The aim of this study is to evaluate the catalytic activity of some minerals and to correlate their physicochemical characteristics with their efficiency in the heterogeneous catalytic ozonation process. For that purpose,five different minerals were used as catalysts: anatase, dolomite, kaolin, talc and zeolite in catalytic ozonation of p-CBA. The most widely used catalyst among them is zeolite [6,7,8], but there are also some researches about anatase [5,9] and kaolin [4,10]. p-CBA was employed as a model compound, because it cannot be efficiently removed by direct ozonation (kO3< 0.15 M−1s−1), while it has high reactivity with hydroxyl radicals (k•OH = 5×109 M−1s−1) [11,12]. As a result, the production of hydroxyl radicals can be indirectly evaluated.
2. Materials and Methods
All the chemicals were of analytical grade, except from HPLC-grade acetonitrile (Chem-lab, Zedelgem, Belgium) and phosphoric acid (Sigma-Aldrich, St. Louis-MO-USA). p-CBA (Sigma-Aldrich, St. Louis-MO-USA) was used as the model organic compound at initial concentration of 4 μM. All the solutions were prepared by distilled water. Anatase, dolomite, kaolin, talc and zeolite were used as catalysts. K2HPO4 and KH2PO4 (Chem-lab, Zedelgem, Belgium) were used for pH adjustment. Adsorption, ozonation and catalytic ozonation experiments were performed in batch mode following the procedure described by Psaltou et. al., 2018 [13]. The residual concentration of p-CBA was determined by HPLC (Thermo, Massachusetts-MA-USA) with UV detector at 254 nm (Thermo, UV2000). The mobile phase was consisted from 60% v/v of 10 mM phosphoric acid and 40% v/v of acetonitrile. The detection limit of p-CBA was 0.025 μΜ. The concentration of ozone in aqueous solutions was determined by common indigo method [14]. The surface area and the pore size distribution of the minerals were calculated, according to the Brunauer–Emmet–Teller (BET) method. The Point of Zero Charge (PZC) was determined by the potentiometric mass titration method [15].
3. Results
In adsorption, the ozonation and catalytic ozonation experiments’ pH was stable at 7, where the p-CBA is negative charged (pKa = 4.1) and the potential catalysts were of different charge: negative (anatase, kaolin, talc), almost neutral (zeolite) and positive (dolomite). The physicochemical characteristics of these catalysts are presented in Table 1.

Table 1.
physicochemical characteristics of minerals.
3.1. Adsorption of p-CBA by Minerals
The adsorption of p-CBA from aqueous solutions by the examined catalysts showed rather low uptake capacity at pH 7 (Table 2). Isoelectric point (IEP) is the main physicochemical characteristic that determines the affinity between the organic molecule and the solid material in adsorption process. Although dolomite was the only mineral that was positively charged throughout adsorption process, its adsorption capacity was very low (12.5 μg p-CBA/g), due to its low specific surface area (Table 1).Talc showed significantly higher uptake capacity than the other catalysts, which was 200.4 μg p-CBA/g. A lower adsorption capacity (9.4 μg p-CBA/g) was determined by kaolin, probably because it has the same IEP value with the pKa of p-CBA.

Table 2.
Adsorption loading of p-CBA (Co = 4 μΜ) onto the minerals (Ccat= 0.5 g/L).
3.2. Heterogeneous Catalytic Ozonation with the Examined Catalysts
The objective of this study was to evaluate the catalytic activity of the examined minerals. Figure 1a shows the results of ozone decomposition in single and catalytic ozonation of p-CBA at pH 7 using the five minerals as catalysts. All the catalysts increasedozone decomposition compared to single ozonation. Higher ozone degradation was presented by dolomite, which consumed 98.5% at 15 min reaction time.Kaolin and zeolite presented a fast consumption at the first 3 min, which reached 89%, and then the ozone consumption slowed down. However, the micropollutant removal did not present the same behavior.
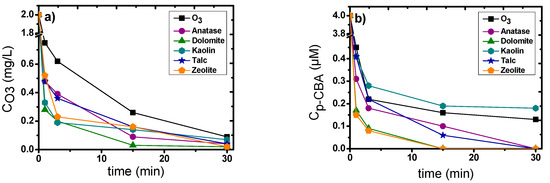
Figure 1.
degradation by heterogeneous catalytic ozonation, (a) Ozone decomposition, (b) p-CBA degradation; experimental conditions: CO3 = 2 mg/L, Cp-CBA = 4 μΜ, Ccat. = 0.5 g/L, pH = 7, T = 23±2°C.
From the results presented in Figure 1b, it is obvious that the highest degradation occurred when dolomite and zeolite were used as catalysts, which can achieve up to 97.8% p-CBA removal already after the 3rd min of reaction/oxidation time. Although these two catalysts did not consume ozone the same way, they presented similar results in p-CBA degradation. However, for almost all examined catalysts, complete degradation of p-CBA (i.e., close to the respective analytical detection limit) was achieved after 30 min of reaction time except from kaolin. As aforementioned, p-CBA is considered an •OH probe compound because it reacts quicklywith hydroxyl radicals and, at the same time, presents very low reactivity against ozone molecules. This indicates that the acceleration of micropollutant degradation was mainly due to the increased production of hydroxyl radicals.
Three of the examined materials (dolomite, talc, zeolite)promoted ozone degradation slowly, as their constants are 0.079 min−1, 0.070 min−1 and 0.074 min−1, respectively (Table 3).Anatase has the highest kinetic constant for ozone decomposition, 0.121 min−1. However, regardless of their differences, all the examined catalysts enhanced ozone decomposition compared with single ozonation (0.037 min−1).

Table 3.
Values of first order kinetic constant (k, min−1) for ozone decomposition without p-CBA in catalytic ozonation systems; Experimental conditions: CO3 = 2 mg/L, Ccat. = 0.5 g/L, pH = 7, T = 23 ± 2 °C.
4. Discussion
The heterogeneous catalytic ozonation of p-CBA by using minerals as catalysts (anatase, dolomite, kaolin, talc, zeolite) in aqueous solutions was found to be capable ofincreasing the removal/degradation of initial p-CBA concentration compared to a single ozonation process except inthe case of kaolin. Since p-CBA degradation is particularly favored by the presence of hydroxyl radicals (and not by single ozonation), it is concluded that four of the examined catalysts can enhance the decomposition of ozone on their reactive sites. The best results were obtained with the presence of dolomite and zeolite at 3 min reaction time, with p-CBA removal up to 97.8%, which increased to 99.4% at 15 min reaction time. The catalytic performance of zeolite was probably due to the neutral charging of its surface (PZC = 6.8) in combination with its adsorption capacity (97.1 μg/g catalyst), which favored the affinity between ozone/hydroxyl radicals and p-CBA. Dolomite has a high point of zero charge at 8.9, and it was positive charged throughout the reaction. On the other hand, p-CBA has pKa 4.1, i.e., it is negative charged at pH 7. The opposite charge creates an affinity between the catalyst and the organic molecule, and as a result, heterogeneous catalytic ozonation is favored. This affinity did not favor the adsorption process, because the specific surface area and the pore volume of dolomite are small (Table 1). Although they present high ozone decomposition constants,anatase and kaolin did not remove p-CBA satisfactorily, because they have low adsorption capacity and the production of hydroxyl radicals are so quick that the produced oxidative species cannot react with the pollutant. Talc with high adsorption capacity and low kinetic constant removed p-CBA by 94.5% at 3 min of reaction due to its negative charge that does not favor the production of hydroxyl radicals. From the results of this study, it is obvious that the catalytic activity and the adsorption capacity of a catalyst are affected by its physicochemical characteristics. Surface charge has the greater effect (PZC and IEP) on which the decomposition of ozone and the adsorption capacity depends.
Author Contributions
S.P. and M.M. conceived and designed the experiments; S.P. performed the experiments; S.P. and E.K. analyzed the data; A.Z. and M.M. contributed reagents/materials/analysis tools; S.P. wrote the paper.
Acknowledgments
This research has been co-financed by the European Union and Greek national funds through the Operational Program Competitiveness, Entrepreneurship and Innovation, under the call RESEACH-CREATE-INNOVATE (project code: T1EDK-02397).


Conflicts of Interest
The authors declare no conflict of interest.
References
- Rodriguez-Narvaez, O.M.; Peralta-Hernandez, J.M.; Goonetilleke, A.; Bandala, E.R. Treatment technologies for emerging contaminants in water: A review. Chem. Eng. J. 2017, 323, 361–380. [Google Scholar] [CrossRef]
- Nawrocki, J.; Kasprzyk-Hordern, B. The efficiency and mechanisms of catalytic ozonation. Appl. Catal. B Environ. 2010, 99, 27–42. [Google Scholar] [CrossRef]
- Kasprzyk-Hordern, B.; Ziółek, M.; Nawrocki, J. Catalytic ozonation and methods of enhancing molecular ozone reactions in water treatment. Appl. Catal. B Environ. 2003, 46, 639–669. [Google Scholar] [CrossRef]
- Ma, W.; Hu, J.; Yoza, A.B.; Wang, Q.; Zhang, X.; Li, X.Q.; Guo, S.; Chen, C. Kaolinite based catalysts for efficient ozonation of recalcitrant organic chemicals in water. Appl. Clay Sci. 2019, 175, 159–168. [Google Scholar] [CrossRef]
- He, Z.; Cai, Q.; Hong, F.; Jiang, Z.; Chen, J.; Song, S. Effective Enhancement of the Degradation of Oxalic Acid by Catalytic Ozonation with TiO2 by Exposure of {001} Facets and Surface Fluorination. Ind. Eng. Chem. Res. 2012, 51, 5662–5668. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Brown, D.R.; Kasprzyk-Hordern, B. Mechanisms of catalytic ozonation on alumina and zeolites in water:Formation of hydroxyl radicals. Appl. Catal. B Environ. 2012, 123–124, 94–106. [Google Scholar] [CrossRef]
- Ikhlaq, A.; Waheed, S.; Joya, K.S.; Kazmi, M. Catalytic ozonation of paracetamol on zeolite: ANon-radical mechanism. Catal. Commun. 2018, 112, 15–20. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, W.; Yoza, B.A.; Xu, Y.; Li, X.Q.; Chen, C.; Wang, Q.; Gao, Y.; Guo, S.; Zhan, Y. Investigation of Catalytic Ozonation of Recalcitrant Organic Chemicals in Aqueous Solution over Various. Catalysts 2018, 8, 128. [Google Scholar] [CrossRef]
- Yang, Y.X.; Ma, J.; Qin, Q.; Zhai, X. Degradation of nitrobenzene by nano-TiO2 catalyzed ozonation. J. Mol. Catal. A Chem. 2007, 267, 41–48. [Google Scholar] [CrossRef]
- Gao, L.; Zhai, Y.; Ma, H.; Wang, B. Degradation of cationic dye methylene blue by ozonation assisted with kaolin. Appl. Clay Sci. 2009, 46, 226–229. [Google Scholar] [CrossRef]
- Pi, Y.; Schumacher, J.; Jekel, M. The use of para-chlorobenzoic acid (pCBA) as an ozone/hydroxyl radical probe compound. Ozone Sci. Eng. 2005, 27, 431–436. [Google Scholar] [CrossRef]
- Huang, R.; Lan, B.; Chen, Z.; Yan, H.; Zhang, Q.; Li, L. Catalytic ozonation of p -chlorobenzoic acid over MCM-41 and Fe loaded MCM-41. Chem. Eng. J. 2012, 180, 19–24. [Google Scholar] [CrossRef]
- Psaltou, S.; Stylianou, S.; Mitrakas, M.; Zouboulis, A. Heterogeneous Catalytic Ozonation of p-Chlorobenzoic Acid in Aqueous Solution by FeMnOOH and PET. Separations 2018, 5, 42. [Google Scholar] [CrossRef]
- Clesceri, S.L.; Greenberg, E.A.; Trussel, R.R. Inorganic Nonmetals. In Standard Methods for Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989; pp. 162–165. ISBN 0-87553-161-X. [Google Scholar]
- Kosmulski, M. Surface Charging and Points Zero Charge; CRC: Boca Raton, FL, USA, 2009; Volume 145, ISBN 978-1-4200-51889-9. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).